The p-Block Elements Important Extra Questions Very Short Answer Type
Question 1.
do boron halides form additional compounds with amines?
Answer:
Boron halides are Lewis acids and hence accept a pair of electrons from amines to form additional compounds.
Question 2.
How does boron interact with NaOH?
Answer:
2B + 6NaOH → 2Na3BO3 + 3H2.
Question 3.
What is the oxidation state of C in
(a) CO
Answer:
+ 2
(b) HCN
Answer:
+ 2
(c) H2CO3
Answer:
+ 4
(d) CaC2.
Answer:
-1.
Question 4.
What is the state of hybridization of C in
(a) CO32-
Answer:
sp2
(b) CCl4
Answer:
sp3
(c) diamond
Answer:
sp3
(d) graphite?
Answer:
sp2
Question 5.
Give two examples of electron-deficient compounds.
Answer:
BF3 and B2H6.
Question 6.
Arrange the following halides of boron in the increasing order of acidic character.
BF3, BCl3, BBr3, BI3.
Answer:
BF3 < BCl3 < BBr3 < BI3.
Question 7.
What is dry ice? Why is it so-called?
Answer:
Solid CO2 is known as dry ice. It does not wet a piece of paper/cloth and sublimes without melting. Therefore, it is called dry ice.
Question 8.
Write balanced equations to show hydrolysis reactions of CO32- and HCO3–.
Answer:
CO32- + H2O ⇌ OH– + HCO3–
HCO3– + H2O ⇌ OH– + H2CO3.
Question 9.
Why boron does not form B3+ ion?
Answer:
Boron has a very high sum of the first three ionisation enthalpies. Hence it cannot lose three electrons to form a B3+ ion.
Question 10.
Which oxide of carbon is an anhydride of carbonic acid?
Answer:
CO2, because H2CO3 acid decomposes to give H2O and CO2.
Question 11.
What happens when a borax solution is acidified? Write a balanced equation for the reaction.
Answer:
Boric acid is formed.
Na2B4O7 + 2HCl + 5H2O → 2NaCl + 4H3BO3 (boric acid)
Question 12.
By means of a balanced equation show how B(OH)3 behaves as an acid in water.
Answer:
B(OH)3 + 2HOH → [B(OH)– + H3O+.
Question 13.
What happens when boric acid is heated?
Answer: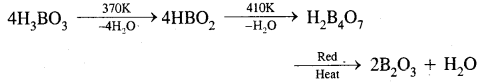
Question 14.
What are boranes?
Answer:
Stable covalent hydrides of boron like B2H6, B4H10, B5H9 on analogy with alkanes are called boranes.
Question 15.
Write a balanced equation for the preparation of boron by reduction of BBr3.
Answer:
2BBr3(g) + 3H2(g) → 2B(s) + 6HBr(g)
Question 16.
What is carborundum? What is its common use?
Answer:
Silicon carbide (SiC). It is used as an abrasive.
Question 17.
What is Freon gas? To what use is it put?
Answer:
Freon gas is dichlorodifluoromethane CCl2F2. It is used as a coolant in refrigerators and in air-conditioners.
Question 18.
Give the name of the compound used as a fire extinguisher under the name pyrone.
Answer:
Carbon tetrachloride (CCl4).
Question 19.
What happens when aluminium metal is dipped in cone, nitric acid?
Answer:
One molecule thick layer of oxide is formed on the surface of A1 and further reaction does not proceed. Al is said to become passive.
Question 20.
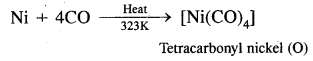
What happens when CO is passed over heated nickel?
Answer:
Ni + 4CO → Ni(CO)4.
Tetra carbonyl nickel (O) is formed.
Question 21.
How does boron react with dinitrogen?
Answer:
2B + N2 → 2BN (boron nitride)
Question 22.
What is the chemical formula of Borazole or borazine? Why is it called inorganic benzene?
Answer:
Borazole is B3N3H6. Because of its similarity to benzene, it is called inorganic benzene.
Question 23.
Write down the structure of Borazole.
Answer:
B3N3H6.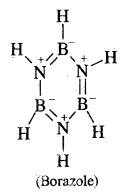
Question 24.
Why thallium prefers to show an oxidation state of +1 rather than +3?
Answer:
Due to the inert-pair effect.
Question 25.
How do you explain that anhydrous AlCl3 is covalent, but hydrated AlCl3 is electrovalent?
Answer:
In the presence of H2O, Al2Cl6 dissociates into hydrated Al3+ and Cl– ions due to the high heat of hydration of these ions.
Question 26.
Why BBr3 is a stronger Lewis acid than BF3?
Answer:
This is because the back donation of electrons into empty 2p orbital of boron atom from filled p orbital of Br atom is much less than that by F atom due to larger size of Br atom than F atom.
Question 27.
Why trihalides of group 13 elements fume in the moist air?
Answer:
They are hydrolysed by water forming hydrogen halides.
MX3 + 3H2O → M(OH)3 + 3HX.
Question 28.
Aluminium forms [AlF6]3-, but boron does not form [BF6]3- why?
Answer:
Boron does not have vacant d-orbitals. Therefore, it cannot expand its coordination number beyond four.
Question 29.
Why boron halides do not exist as dimers whereas AlCl3 exists as Al2Cl6?
Answer:
Boron atom being small iff size is unable to accommodate large-sized halogens around it.
Question 30.
Gold has much higher first ionisation energy than boron, yet gold is metal while boron is non-metal. Explain.
Answer:
This is based on their crystal structure. Gold has a coordination number of 12 while boron has 6 or less than 6.
Question 31.
Why CO2 is a gas whereas SiO2 is solid. Explain why?
Answer:
CO2 forms monomeric linear molecules, while SiO2 exists as a giant-sized 3-dimensional network structure.
Question 32.
C and Si are almost tetravalent, but Ge, Sn, Pb show divalency. Why?
Answer:
The inert pair effect is shown by Ge, Sn, Pb.
Question 33.
Account for the fact that PbX2 is more stable than PbX4. [X = Cl, Br]
Answer:
Due to the inert pair effect, Pb shows an oxidation state of +2.
Question 34.
PbCl4 is less stable than SnCl4, but PbCl2 is more stable than SnCl2. Why?
Answer:
Stability of + 4 oxidation state decreases down the group while that of + 2 oxidation state increases due to inert pair effect.
Question 35.
Why carbon shows maximum catenation in group 14 elements?
Answer:
Due to the strong C-C bond, its bond dissociation energy is the highest among group 14 members,
Question 36.
Pyrosilicates contain which anion?
Answer:
Si2O76-.
Question 37.
Mention one industrial application of silicones.
Answer:
Silicones are used for making water-proof papers by coating them with a thin layer of silicones.
Question 38.
What is the basic building unit of silicates?
Answer:
SiO44- tetrahedra.
Question 39.
Which element of group 13 forms amphoteric hydroxide?
Answer:
Aluminium.
Question 40.
Which element of group 13 forms the most stable oxidation state of + 1?
Answer:
Thallium.
Question 41.
Explain why silicon shows a higher covalency than carbon?
Answer:
Due to the presence of d-orbitals, silicon shows a higher covalency of 6.
Question 42.
What are silicones?
Answer:
Silicones are synthetic organosilicon compounds containing repeated R2SiO units held by Si—O—Si linkages.
Question 43.
What are the 2 main uses of zeolites?
Answer:
- Softening of hard water.
- Catalysts in petrochemical industries.
Question 44.
Why is boric acid considered a weak acid?
Answer:
Because it is not able to release H+ ions on its own. It receives OH– ions from the water molecule to complete its octet & in turn releases H+ ions.
Question 45.
[SiF6]-2 is known whereas [SiCl6]2- not. Why?
Answer:
The reasons are:
- Six large chlorine atoms cannot be accommodated around the Silicon atom due to the limitation of its size.
- Interaction between lone pair of chlorine atom & silicon atom is not very strong.
Question 46.
Diamond is co-valent, yet it has a high M.P. Why?
Answer:
Diamond has a three-dimensional networked structure involving a strong C—C bond, which are very difficult to break & in turn it has a high melting point.
Question 47.
What is the common name of the recently developed allotrope of carbon i.e. C60 molecule?
Answer:
Fullerene.
Question 48.
The M.P. & B.P. of Carbon & Silicon is very high. Why?
Answer:
This is due to the tendency of these elements to form giant molecules.
Question 49.
Why is boron metalloid?
Answer:
Boron resembles both metals & non-metals therefore it is metalloid.
Question 50.
Why does boron resemble Si?
Answer:
Both have a similar charge to radius ratio, i.e. similar polarizing power.
The p-Block Elements Important Extra Questions Short Answer Type
Question 1.
Although boric acid B(OH)3 contains three hydroxyl groups, yet it behaves as a monobasic acid. Explain.
Answer: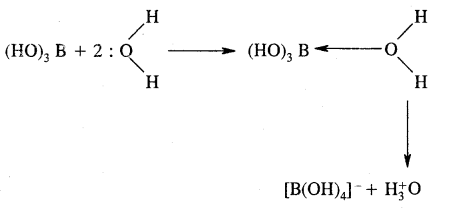
Hydrated species
B(OH)3 is not a protonic acid.
It behaves as a Lewis acid because it abstracts a pair of electrons from hydroxyl ion.
Question 2.
SiCl4 forms [SiCl6]2- while CCl4 does not form [CCl6]2- Explain.
Answer:
Carbon does not have d-orbitals and hence C.Cl4 does not combine with Cl– ions to give [CCl6]2-, On the other hand, silicon has vacant 3d-orbitals and thus can expand its covalency from 4 to 6. Therefore SiCl4 combines with CL ions to form [SiCl6]2-
SiCl4 + 2Cl– → [SiCl6]2-
Question 3.
Why does not silicon form an analogue of graphite?
Or
Why does elemental silicon not form a graphite-like structure as carbon does? Explain.
Answer:
In graphite, C is sp2 hybridised and each C is linked to three other C atoms forming hexagonal rings. Thus graphite has a two-dimensional sheet-like structure.
Silicon, on the other hand, does not form an analogue of C because of the following two reasons:
- Silicon has a much lesser tendency for catenation than C as Si-Si bonds are much weaker than C-C bonds.
- Silicon because of its larger size than C undergoes sp3 hybridisation.
Question 4.
Why carbon forms covalent compounds whereas lead forms ionic compounds?
Answer:
Carbon cannot lose electrons to form C4 because the sum of four ionisation enthalpies is very high. It cannot gain four electrons to form C4 because energetically it is not favourable. Hence C forms only covalent compounds. Down the group 14, ionisation enthalpies decrease, Pb being the last element has so low I.E. that it can lose electrons to form ionic compounds.
Question 5.
How is borax prepared from
(i) Colemanite ore
Answer:
Borax is also called sodium tetraborate decahydrate (Na2B4O7.10H2O). It can be prepared as follows:
From colemanite: Powdered mineral is boiled with sodium carbonate solution and filtered. The filtrate is concentrated and then cooled when crystals of borax.
Ca2B6O11 + 2Na2CO3 → Na2B4O7 + 2NaBO2 + 2CaCO3.
The mother-liquor which contains sodium meta-borate is treated with a current of C02, to convert it into borax which separates out.
4NaBO2 + CO2 → Na2B4O7 + Na2CO3
(ii) Tincal.
Answer:
From Tincal: Tincal obtained from dried up lakes is boiled with water. The solution is filtered to get rid of insoluble impurities of clay, sand etc. The filtrate is concentrated to get the crystals of borax.
(iii) Boric acid?
Answer:
From boric acid: Boric acid is neutralised with sodium carbonate and the resulting solution is concentrated and cooled to get the crystals of borax Na2B4O7.10H2O.
4H3BO3 + Na2CO3 → Na2B4O7 + 6H2O + CO2 ↑
Question 6.
Mention three important uses of borax
Answer:
It is used:
- As a flux soldering and welding in industry.
- In the manufacture of borosilicate glass (or pyrex glass).
- In making enamels and glazes.
- In stiffening of candle wicks.
- In softening of water.
- In a qualitative analysis for borax bead test in the laboratory.
Question 7.
What happens when a borax solution is acidified? Write a balanced equation for the reaction.
Answer:
When acidified aqueous solution of borax (Na2B4O7) is heated, boric acid is formed.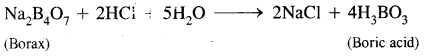
Question 8.
Mention important uses of boric acid.
Answer:
Boric acid is used:
- In the manufacture of enamels and glazes for pottery.
- In making heat-resisting and shock resisting glass called boro glass (or pyrex glass).
- As a mild antiseptic for washing eyes
Question 9.
Mention some important properties of carbon monoxide.
Answer:
- It is a colourless, odourless gas, slightly soluble in water.
- It is highly poisonous. It combines with haemoglobin in the red blood cells to form carboxy-haemoglobin which cannot absorb oxygen and thus the supply of oxygen to the body is reduced.
- It burns with a pale blue flame forming CO2.
2CO + O2 → 2CO2. - It is a reducing agent. It reduces some metal oxides into metals.
Fe2O3 + 3CO → 2Fe + 3CO2. - It combines With transition metals like iron, cobalt, nickel to form their carbonyl compounds.
Question 10.
What are halides of carbon? Give few examples.
Answer:
Carbon combines with halogens to form both simple and mixed tetrahalides. In the case of simple halides, all the four expected tetrahalides (e.g. CF4, CCl4, CBr4 and Cl4) are known to exist. The stability of the simple tetrahalides decreases with the increasing atomic mass of the halogen.
(CF4 > CCl4 > CBr4 > Cl4)
Amongst the mixed halides the better-known compounds are (CFCl3, CF2Cl2 and C Cl3Br).
Question 11.
What is allotropy? Give examples of allotropes.
Answer:
Two or more forms of the same element in the same physical state which differ in their physical properties but have the same chemical properties are called allotropic forms or (allotropes) and the phenomenon is called allotropy.
Carbon, phosphorus and sulphur are some elements that exhibit allotropy.
- Diamond and graphite are allotropic forms of carbon.
- Red phosphorus and white .phosphorus are allotropes of phosphorus.
- Rhombic sulphur, monoclinic sulphur and plastic sulphur are allotropic forms of sulphur.
Question 12.
Give the uses of different allotropic forms of carbon.
Answer:
| Forms of carbon | Uses |
| Diamond | Gemstone, cutting, drilling, grinding, polishing, industry. |
| Graphite | Reducing agent, refractories, pencils, high-temperature crucibles, electrode making, the moderator in nuclear reactors, high strength composite materials/ |
| Activated carbon | Rubber industry, pigments in ink, paints and plastics. |
| Coke | Fuel, strut manufacture. |
| Charcoal | Fuel, reducing agent. |
Question 13.
Give reasons:
(i) Graphite is used as a lubricant.
Answer:
Graphite has sp3 hybridized carbon with a layer structure. Due to wide separation & weak interlayer bonds, the two adjacent layers can easily slide over each other. This makes graphite act as a lubricant.
(ii) Cone. HN03 can be transported in an aluminium container.
Answer:
Aluminium on coming in contact with the cone; HNO3 becomes passive due to the coating of aluminium oxide & thus the cone. HNO3 can be transported in an aluminium container.
(iii) Aluminium utensils should not be kept in water overnight.
Answer:
Aluminium utensils should not be kept in water overnight because aluminium is readily corroded by water.
Question 14.
Write balanced equations for the reaction of elemental boron with elemental chlorine, oxygen & nitrogen at a high temperature.
Answer: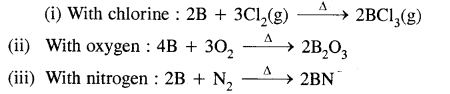
Question 15.
Why are boron halides & diborane referred to as “Electron deficient compounds”?
Answer:
Boron in its halides has only six electrons in its valence shell, therefore it is short by two electrons to complete its octet. As a result, a molecule of boron halide can accept a pair of electrons from any electron-rich compound that is why boron halides are called electron-deficient compounds. In diborane, the total no. of valence electrons is not sufficient to completely fill the. available orbitals. This gives an electron-deficient character to diborane.
Question 16.
Give reasons:
(i) A mix. of dil. NaOH & aluminium pieces are used to open drains.
Answer:
Aluminium dissolves in dil. NaOH with the evolution of H2. This H2 helps to open the drain.
(ii) Diamond is used as an abrasive.
Answer:
Diamond is the hardest substance known & thus used as abrasive & for cutting glass.
(iii) Aluminium wires are used to make transmission cables.
Answer:
Aluminium is cheaply available on a weight to weight basis, the electrical conductivity of aluminium is twice that of copper. Hence Aluminium wires are used to make transmission cables.
Question 17.
Write balanced equations:
(i) B2H6 + NH3 →?
Answer: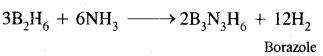
(ii) NaH+ B2H6 →?
Answer: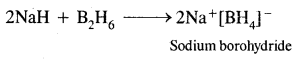
(iii) BF3 + LiH →?
Answer: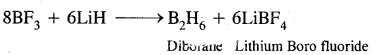
Question 18.
Describe briefly three different methods of obtaining elemental boron.
Answer:
- By reduction of oxides: The reduction of oxides is carried out by electropositive metals like Mg.
B2O3(s) + 3Mg(s) → 2B(s) + 3MgO(s) - By reduction of halides: The reduction of the volatile boron halides is carried but by dihydrogen at high temperatures
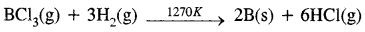
- By the thermal decomposition of boron hydride
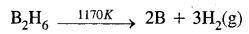
Question 19.
All the elements of group 13 except thallium show a +3 oxidation state while it shows a +1 oxidation state. Give reasons.
Answer:
The valence shell of group 13-elements have two electrons in s-subshell & 1-electron in p-subshell Therefore, they are expected to show a +3 oxidation state. In thallium, the electrons of the s-subshell do not take part in bond formation due to the inert pair effect & only one electron of the p-orbital participate in bond formation, thus it shows +1 oxidation state only.
Question 20.
Why carbon does not form ionic compounds?
Answer:
The electronic configuration of carbon atom is 1s2, 2s2, 2px’, 2py’ & has four valence electrons. In order to form ionic compounds, it has to either lose four electrons or gain four electrons. Since very high energies are involved in doing so. Thus carbon does not form ionic compounds. It completes its octet as a result of electron sharing & forms covalent compounds.
Question 21.
Gallium has higher ionization enthalpy than Aluminium. Explain.
Answer:
Gallium has higher ionization energy than Aluminium because of the higher effective nuclear charge. This is due to additional 3d electrons which do not screen the nuclear charge effectively so that the outer electrons are more strongly held.
Question 22.
Boron forms no compounds in a unipositive state but thallium in a unipositive state is quite unstable. Why?
Answer:
Boron has electronic configuration 2s22p1 & therefore forms compounds in a trivalent state. However, thallium prefers to form compounds in the +1 oxidation state rather than in the +3 oxidation state as suggested by its group number. This is due to the inert pair effect. According to this effect, the 6s2 electrons in the case of heavy metals preferably do not take part in bonding.
Question 23.
What are silicones? How are they manufactured?
Answer:
Silicones are rubber-like polymers having Si – O-Si linkage & general formula R2SiO. They are manufactured by hydrolysis of Chloro silicones,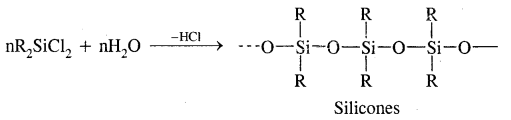
Where R is Me/Ph groups
Question 24.
Mention any two dissimilarities of boron with other elements of group-13.
Answer:
- All the compounds of boron are covalent in nature because of the non-existence of the B3+ ion. It is because of its high charge density.
- The maximum covalence shown by Boron is 4 while other members of this group show a covalence of six or more.
Question 25.
Why boron trihalides form tetrahedral complexes?
Answer:
A Boron trihalide is an electron-deficient compound having six electrons in its outermost shell. Therefore it has a tendency to form a BX3L type of complex after accepting an electron pair in the un-hybridized vacant p-orbital from a ligand (L) molecule. Because of this reason the shape of BX3 changes from planar Triangular to tetrahedral in the BX3L complex.
Question 26.
Discuss the pattern of variation in the oxidation states of Al & Tl.
Answer:
Aluminium shows a +3 oxidation state only while thallium, the last element of group 3 shows +3 oxidation state & +1 oxidation states. Tl+1 is more stable than Tl3+ as is evident by redox potential data
Tl3+(aq) + 2e– → Tl+ (aq) E° = +1.25 V
It happens because of the decrease in bond energy with size down the group (i.e. from Al → Tl). Thus the energy required to break the pair of ns2 electrons is not compensated by the energy released during the formation of two additional bonds. Thus +1 oxidation state is more stable in thallium.
Question 27.
C—C bond length is shorter in graphite than the C-C bond length of diamond. Explain.
Answer:
Graphite has sp2 hybridization & the C-C bond involves sp2—sp2 hybridized carbon. Diamond has sp3 hybridization & the C—C bond involves sp3-sp3 hybridization. Furthermore, the more the S character in a hybridized atom, the smaller is the size of the hybridized orbital, resulting in more overlapping which leads to bond length.
Question 28.
[SiF6]2- is known whereas [SiCl6]2- not. Give reasons.
Answer:
- Six large chlorine atoms cannot be accommodated around silicon atom due to the limitation of their size.
- Interaction between lone pair of chlorine atoms.& silicon atom is not very strong.
Question 29.
SiCl. forms [SiCl]2- while CClL does not form [CCl]2-. Explain
Answer:
Carbon does not have d-orbitals, hence CCl4 does not combine with Cl ions to form [CCl6]2-. On the other hand, silicon has vacant 3-d orbitals & thus SiCl4 combines with Cl– ions to form
[Sicy2-
SiCl4 + 2Cl– → [SiCl6]2-
In other words, carbon shows a fixed covalency of 4 but silicon exhibits varying covalency from 4 to 6.
Question 30.
Borazine is more reactive than benzene. Why?
Answer:
Both Borazine & Benzene are isoelectronic. In benzene C = C bonds are non-polar while N=B bonds in borazine are polar in nature due to the presence of a co-ordinate bond between N & B atoms. As a result, addiction is quite frequent in borazine while it is less in benzene because of delocalisation of π-electron charge.
The p-Block Elements Important Extra Questions Long Answer Type
Question 1.
(i) What are the different oxidation states exhibited by the group 14 elements? Discuss the stability of their oxidation states.
Answer:
The group 14 elements have four electrons in the outermost shell. The common oxidation states exhibited by these elements are +4 and +2. Since the sum of the first four ionisation enthalpies is very high, compounds in the +4 oxidation state are generally covalent in nature. In heavier members such as Ge, Sn and Pb, the tendency to show +2 oxidation state increases. It is due to the inability of ns2 electrons of the valence shell to participate in bonding.
The relative stabilities of these two oxidation states vary down the group. C and Si mostly show a +4 oxidation state. Ge forms stable compounds in the +4 state and only a few compounds in the +2 state. Sn forms compounds in both oxidation states (Sn in +2 state is a reducing agent).
Lead compounds in the +2 state are stable and in the +4 state are strong oxidising agents. In the tetravalent state, the number of electrons around the central atom in a molecule (e.g., carbon in CCl4) is eight. Being electron precise molecules, they are normally not expected to act as an electron acceptor or electron donor.
Although carbon cannot exceed its covalence of more than 4, other elements of the group can do so. It is because of the presence of d-orbital in them. Due to this, their halides undergo hydrolysis and have a tendency to form complexes by accepting electron pairs from donor species. For example, the species like SiF5–, SiF6–, [GeCl6]2-, [Sn(OH)6]2- exist where the hybridisation of the central atom is sp3d2.
(ii) What type of oxides are formed by group 14 elements? Which of them are acidic, neutral or basic?
Answer:
All members when heated in oxygen form oxides. There are -mainly two types of oxides, i. e., monoxide and dioxide of formula MO and MO2 respectively. SiO only exists at high temperature. Oxides in the higher oxidation state of elements are generally more acidic than those in the lower oxidation state. The dioxides-CO2, SiO2 and GeO2 are acidic, whereas SnO2 and PbO2 are amphoteric in nature. Among monoxides, CO is neutral, GeO is distinctly acidic whereas SnO and PbO are amphoteric.
Question 2.
(a) [SiF6]2- is known whereas [SiCl6]2- is not known. Give reasons
Answer:
(i) [SiF6]2- is known whereas [SiCl6]2- does not exist.
The main reasons are (i) six large chlorine atoms cannot be accommodated around silicon atom due to the limitation of their size.
(ii) Interactions between lone pairs of a chlorine atom and silicon atom are not very strong.
(b) Select the member (s) of group 14 that
(i) forms the most acidic oxide
Answer:
The most acidic dioxide is formed by carbon (CO2).
(ii) is commonly found in the +2 oxidation state
Answer:
Lead is mostly found in the +2 oxidation state in its compounds.
(iii) used as a semi-conductor.
Answer:
Silicon and germanium are used as semiconductors.
(c) Explain why a diamond that is covalent has a high melting point?
Answer:
Though diamond has covalent bonding in it, yet it has a high melting point, because a diamond has a 3-dimensional network involving strong C—C bond, which are very difficult to break and in turn, it has a high melting point.
(d) Discuss the reaction of silica with
(i) NaOH
Answer:
SiO2 reacts with HF as follows:
SiO2 + 2NaOH → Na2SiO3 + H2O
(ii) HE
Answer:
SiO2 reacts with HF as follows:
SiO2 + 4HF → SiF4 + 2H2O.
Question 3.
(a) Carbon exhibits catenation, whereas silicon does not. Explain.
Answer:
Carbon shows catenation because of its smaller size, high bond energy of C – C bond, the possibility of sp, sp2, sp3 hybridisation and formation of multiple bonds C-C (1σ), C = C (1σ + 1π),- C = C (1σ + 2π). On the other hand, silicon shows only limited catenation because of its large atomic radius, low bond energy of Si-Si bond and absence of multiple bonds between Si atoms.
(b) How does boron differ from aluminium.
Answer:
Difference between boron and aluminium:
- Boron is a non-metal but aluminium is a metal.
- Boron is a semi-conductor while aluminium is a good conductor of electricity.
- Boron forms a number of hydrides called boranes, but Al forms a polymeric hydride.
- Halides of boron (except BF3) are readily hydrolysed by water whereas halides of A1 are only partially hydrolysed by water.
- B2O3 is acidic, but Al2O3 is amphoteric.
- Boron hydroxide B(OH)3 is acidic, but Al(OH)3 is amphoteric.
(c) Write the similarities between boron and silicon.
Answer:
Similarities between boron and silicon:
- Both are non-metals.
- Both are semi-conductors
- Boron and silicon form a number of covalent hydrides which have similar properties. For example, they spontaneously catch fire on exposure to air and are readily hydrolysed by water.
- The halides of boron and silicon are readily hydrolysed by water.
- Boron trioxide (B2O3) and silicon dioxide (SiO2) are acidic in nature. These dissolve in alkali solution forming borates and silicates.










