Important Questions for Class 12 Physics Chapter 11 Dual Nature of Radiation and Matter Class 12 Important Questions
Dual Nature of Radiation and Matter Class 12 Important Questions Very Short Answer Type
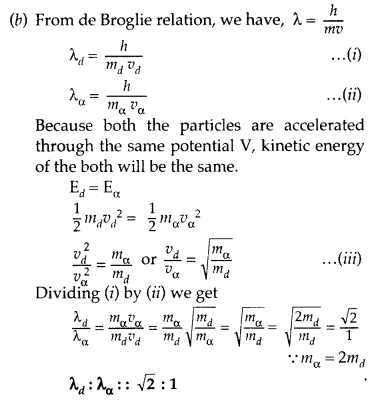
Question 1.
An electron and alpha particle have the same de-Broglie wavelength associated with them. How are their kinetic energies related to each other? (Delhi 2008)
Answer:
Question 2.
Two lines, A and B, in the plot given below show the variation of de-Broglie wavelength, λ versus 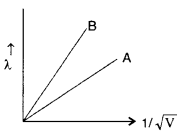
Answer:
Question 3.
The figure shows a plot of three curves a, b, c, showing the variation of photocurrent vs. collector plate potential for three different intensities I1, I2 and I3 having frequencies V1, v2 and v3 respectively incident on a photosensitive surface.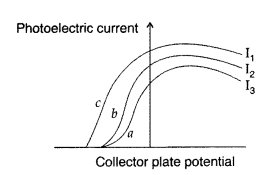
Point out the two curves for which the incident radiations have same frequency but different intensities.
Answer:
Stopping potential will be same for the same frequency. So its curves ‘a’ and ‘b’ which have same frequency but different intensities. (I2 > I3)
Question 4.
The stopping potential in an experiment on photoelectric effect is 1.5 V. What is the maximum kinetic energy of the photoelectrons emitted? (All India 2008)
Answer:
K.E. of the electron e– = 1.5 eV
Question 5.
The maximum kinetic energy of a photoelectron is 3 eV. What is its stopping potential? (All India 2008)
Answer:
Question 6.
Show graphically, the variation of the de- Broglie wavelength (λ) with the potential (V) through which an electron is accelerated from rest.
Answer: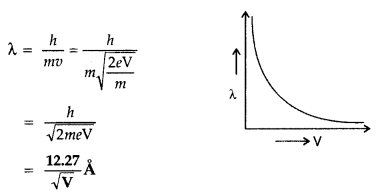
Question 7.
Define the term ‘stopping potential’ in relation to photoelectric effect. (All India 2011)
Answer:
The value of the retarding potential at which the photo electric current becomes zero is called cut off or stopping potential for the given frequency of the incident radiation.
Question 8.
State de-Broglie hypothesis. (Delhi 2011)
Answer:
According to de-Broglie hypothesis, a particle of mass on moving with given velocity v must be associated with a matter waver of wavelength X given by:![]()
Question 9.
A proton and an electron have same kinetic energy. Which one has greater de-Broglie wavelength and why? (All India 2011)
Answer: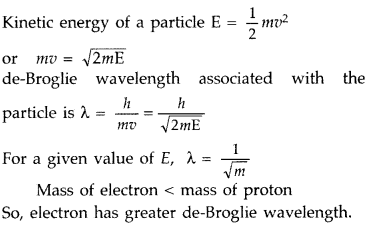
Question 10.
A proton and an electron have same kinetic energy. Which one has smaller de-Broglie wavelength and why? (All India 2011)
Answer:
Question 11.
Define ‘intensity’ of radiation in photon picture of light. (Comptt. Delhi 2011)
Answer:
It is the number of photo electrons emitted per second.
Question 12.
Why is photoelectric emission not possible at all frequencies? (Comptt. All India 2011)
Answer:
Photoelectric emission is possible only if the energy of the incident photon (hv) is greater than the work function (ω0 = hv0) of the metal. Hence the frequency v of the incident radiation must be greater than the threshold frequency v0.
Question 13.
The given graph shows the variation of photo-electric current (I) versus applied voltage (V) for two different photosensitive materials and for two different intensities of the incident radiation. Identify the pairs of curves that correspond to different materials but same intensity of incident radiation. (Delhi 2013)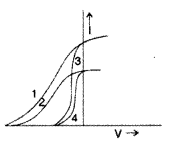
Answer:
The pairs (2, 4) and (1, 3) have same intensity but different material.
Question 14.
Write the expression for the de Broglie wavelength associated with a charged particle having charge ‘q’ and mass ‘m’, when it is accelerated by a potential V. (All India 2013)
Answer: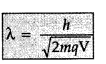
Question 15.
Show on a plot the nature of variation of photoelectric current with the intensity of radiation incident on a photosensitive surface. (Comptt. Delhi 2013)
Answer: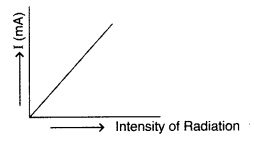
Question 16.
Figure shows a plot of 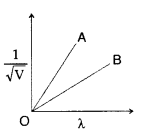
Answer:
B line represents particle of larger mass because slope
Question 17.
Find the ratio of de-Broglie wavelengths associated with two electrons accelerated through 25 V and 36 V. (Comptt. All India 2013)
Answer: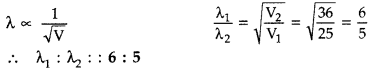
Question 18.
Define intensity of radiation on the basis of photon picture of light. Write its S.I. unit. (All India 2014)
Answer:
It is the number of photo-electrons emitted per second per unit area.
SI unit : m-2S-1
Question 19.
The graph shows the variation of stopping potential with frequency of incident radiation for two photosensitive metals A and B. Which one of the two has higher value of work- function? Justify your answer. (All India 2014)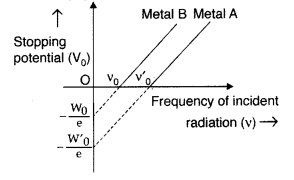
Answer:
Metal ‘A’, because of higher threshold frequency for it.
Question 20.
The graph shows variation of stopping potential V0 versus frequency of incident radiation v for two photosensitive metals A and B. Which of the two metals has higher threshold frequency and why? (All India 2014)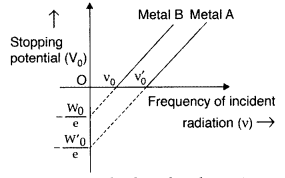
Answer:
Metal ‘A’, because of higher threshold frequency for it.
Question 21.
An electron is revolving around the nucleus with a constant speed of 2.2 × 108 m/s. Find the de-Broglie wavelength associated with it. (Comptt. Delhi 2014)
Answer: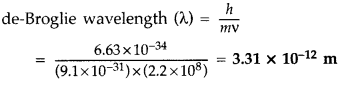
Question 22.
Draw a plot showing the variation of de Broglie wavelength of electron as a function of its K.E.
(Comptt. Delhi 2014)
Answer: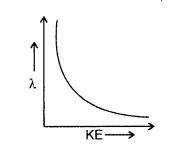
Question 23.
Name the phenomenon which shows the quantum nature of electromagnetic radiation. (Delhi 2014)
Answer:
Photoelectric Effect is the phenomenon which shows the quantum nature of electro-magnetic radiation.
Question 24.
State one factor which determines the intensity of light in the photon picture of light. (Comptt. Delhi 2014)
Answer:
The factor determining the intensity of light is number of electrons emitted per second.
Question 25.
State one reason to explain why wave theory of light does not support photoelectric effect. (Comptt. Delhi 2014)
Answer:
One reason why wave theory of light does not support photoelectric effect is that the kinetic energy of photo electrons does not depend on the intensity of incident light.
Question 26.
If the distance between the source of light and the cathode of a photo cell is doubled, how does it affect the stopping potential applied to the photo cell? (Comptt. Delhi 2014)
Answer:
Stopping potential remains unchanged, if the distance between the light source and cathode is doubled.
Dual Nature of Radiation and Matter Class 12 Important Questions Short Answer Type SA-I
Question 27.
An electron is accelerated through a potential difference of 100 volts. What is the de-Broglie wavelength associated with it? To which part of the electromagnetic spectrum does this value of wavelength correspond? (Delhi 2010)
Answer:
Given : V = 100 V
According to de-Broglie ivavelength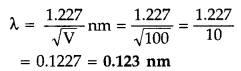
The value of de-Broglie wavelength is 0.123 nm which corresponds to the wavelength of X-rays region of the electromagnetic spectrum.
Question 28.
An electron is accelerated through a potential difference of 64 volts. What is the de-Broglie wavelength associated with it? To which part of the electromagnetic spectrum does this value of wavelength correspond? (Delhi 2010)
Answer:
According to de-Broglie wavelength,![]()
This wavelength is associated with X-rays.
Question 29.
An a-particle and a proton are accelerated from rest by the same potential. Find the ratio of their de-Broglie wavelengths. (All India 2010)
Answer:
de-Broglie wavelength of a charged (q)
Particle accelerated through a potential ‘V’ is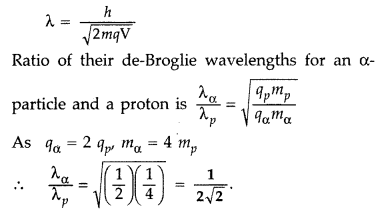
Question 30.
Write Einstein’s photoelectric equation. State clearly the three salient features observed in photoelectric effect, which can be explained on the basis of the above equation. (All India 2010)
Answer:
Einstein’s photoelectric equation is Kmax = hv – ϕ0
(i) We find Kmax depends linearly on V only. It is independent of intensity of radiation.
(iii) Greater the number of energy quanta, greater is the number of photoelectrons. So, photoelectric current is proportional to intensity.
Question 31.
Plot a graph showing the variation of stopping potential with the frequency of incident radiation for two different photosensitive materials having work functions W1 and W2 (W1 > W2). On what factors does the
(i) slope and
(ii) intercept of the lines depend? (Delhi 2010)
Answer: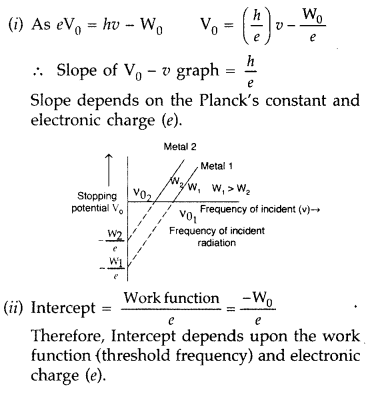
Question 32.
A proton and a deuteron are accelerated through the same accelerating potential. Which one of the two has
(a) greater value of de-Broglie wavelength associated with it, and
(b) less momentum?
Give reasons to justify your answer. (Delhi 2010)
Answer:
For proton and deuteron, charge (q) is the same, while the mass of deuteron is more than that of proton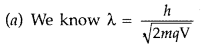
Here q and V are the same for both,![]()
∴ Proton will be associated with greater value of de-Broglie wavelength.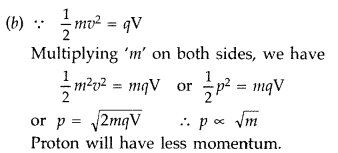
Question 33.
A proton and an alpha particle are accelerated through the same potential. Which one of the two has
(i) greater value of de-Broglie wavelength associated with it, and
(ii) less kinetic energy.
Give reasons to justify your answer. (Delhi 2010)
Answer:
Similar to Q. 32, Page 255
[Hint. Proton’s mass is less than that of alpha particle, which contains 2 protons and 2 neutrons.]
Question 34.
A deuteron and an alpha particle are accelerated with the same accelerating potential. Which one of the two has
(1) greater value of de-Broglie wavelength, associated with it, and
(2) less kinetic energy? Explain. (Delhi 2010)
Answer:
Similar to Q. 32, Page 255
[Hint. A deuteron (consisting of one proton and one neutron) has less mass than alpha particle (consisting of 2 protons and 2 neutrons)]
Question 35.
(i) Monochromatic light of frequency 6.0 × 1014 Hz is produced by a laser. The power emitted is 2.0 × 10-3 W. Estimate the number of photons emitted per second on an average by the source.
(ii) Draw a plot showing the variation of photoelectric current versus the intensity of incident radiation on a given photosensitive surface. (Delhi 2010)
Answer:
(ii)
Question 36.
Two monochromatic radiations of frequencies v1 and v2 (V1 > v2) and having the same intensity are, in turn, incident on a photosensitive surface to cause photoelectric emission. Explain, giving reason, in which case
(i) more number of electrons will be emitted and
(ii) maximum kinetic energy of the emitted photoelectrons will be more. (Comptt. Delhi 2010)
Answer: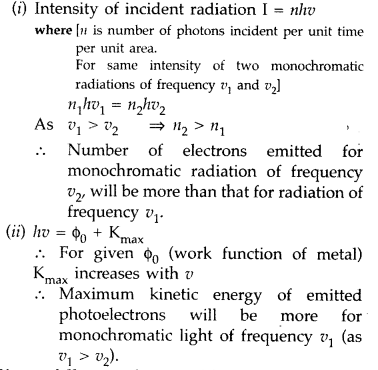
Question 37.
X-rays fall on a photosensitive surface to cause photoelectric emission. Assuming that the work function of the surface can be neglected, find the relation between the de-Broglie wavelength (λ) of the electrons emitted to the energy (E0) of the incident photons. Draw the nature of the graph for X as a function of Ev. (Comptt. Delhi 2010)
Answer: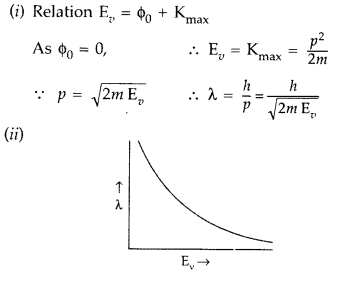
Question 38.
Write three basic properties of photons which are used to obtain Einstein’s photoelectric equation. Use this equation to draw a plot of maximum kinetic energy of the electrons emitted versus the frequency of incident radiation. (Comptt. All India 2010)
Answer:
Properties.
Einstein’s photoelectric equation is Kmax = hv – ϕ0
(i) We find Kmax depends linearly on V only. It is independent of intensity of radiation.
(iii) Greater the number of energy quanta, greater is the number of photoelectrons. So, photoelectric current is proportional to intensity.
Plot of maximum kinetic energy vs. frequency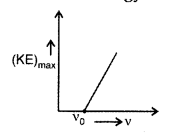
Question 39.
(i) Define the term ‘threshold frequency’ as used in photoelectric effect.
(ii) Plot a graph showing the variation of photoelectric current as a function of anode potential for two light beams having the same frequency but different intensities I1 and I2 (I1 > I2). (Comptt. All India 2010)
Answer:
(i) Threshold frequency. The minimum frequency v0 which the incident light must possess so as to eject photoelectrons from a metal surface, is called threshold frequency of the metal.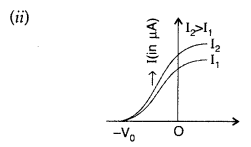
Question 40.
A proton and an a-particle have the same de- roglie wavelength. Determine the ratio of
(i) their accelerating potentials
(ii) their speeds. (Delhi 2015)
Answer:
The de-Broglie wavelength for a proton is,
Question 41.
Using the graph shown in the figure for stopping potential v/s the incident frequency of photons, calculate Planck’s constant. (Comptt. Delhi 2015)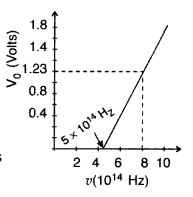
Answer: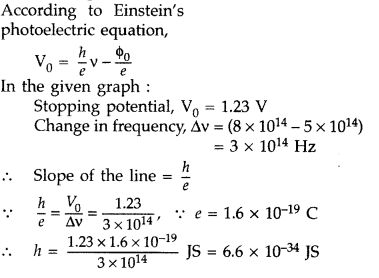
Question 42.
Plot a graph showing variation of de-Broglie wavelength λ versus
Answer: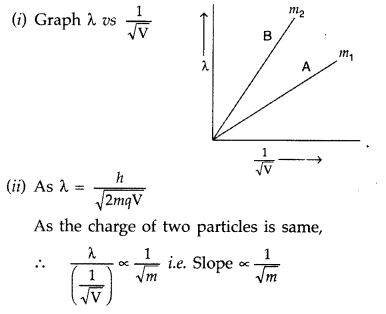
Hence, particle with lower mass (m2) will have greater slope and is represented by the graph ‘B’.
Question 43.
Calculate the de-Broglie wavelength of the electron orbitting in the n = 2 state of hydrogen atom. (All India 2015)
Answer:
Given : n = 2 of hydrogen atom X = ?
Kinetic energy for the second state,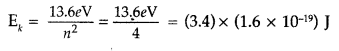
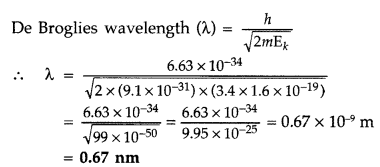
Question 44.
The work function (W), of a metal X, equals 3 × 10-19 J. Calculate the number (N) of photons, of light of wavelength 26.52 nm, whose total energy equals W. (Comptt. Delhi 2015)
Answer: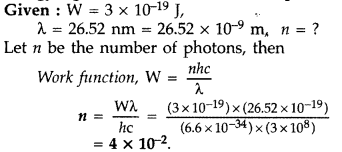
Question 45.
The Kinetic Energy (K.E.), of a beam of electrons, accelerated through a potential V, equals the energy of a photon of wavelength 5460 nm. Find the de Broglie wavelength associated with this beam of electrons. (Comptt. All India 2015)
Answer: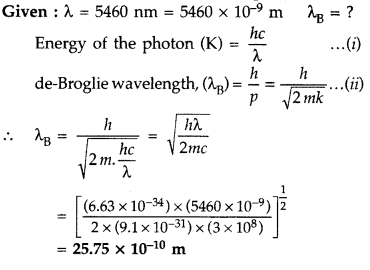
Question 46.
An a-particle and a proton are accelerated through the same potential difference. Find the ratio of their de Broglie wavelengths. (Delhi 2015)
Answer:
From de Broglie equation, we know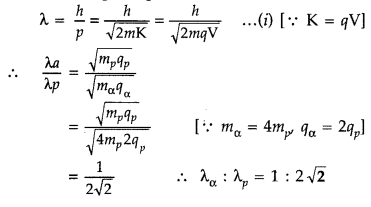
Question 47.
Electrons are emitted from the cathode of a photocell of negligible work function, when photons of wavelength are incident on it. Derive the expression for the de Broglie wavelength of the electrons emitted in terms of the wavelength of the incident light. (Comptt. All India 2015)
Answer: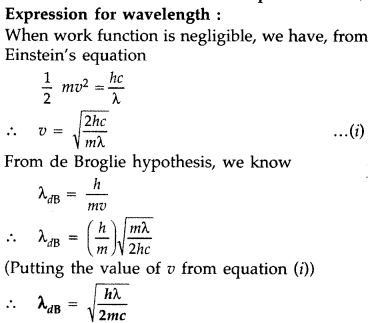
Question 48.
Derive the Bohr’s quantisation condition for angular momentum of the orbitting of electron in hydrogen atom, using de Broglie’s hypothesis. (Comptt. All India 2015)
Answer: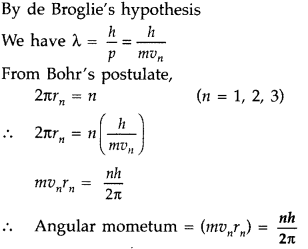
Question 49.
Calculate the kinetic energy of an electron having de Broglie wavelength of 1Å. (Comptt. All India 2015)
Answer: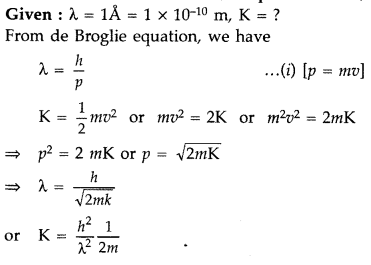
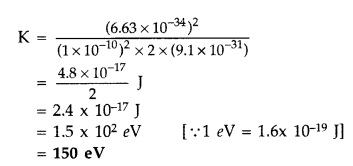
Question 50.
State two properties of photons. For a monochromatic radiation incident on a photosensitive surface, why do all photoelectrons not come out with the same energy? Give reason for your answer.
(Comptt. All India 2017)
Answer:
- Two properties of photons :
- photon is electrically neutral.
- photon has an energy equal to hv
- For a monochromatic radiation incident on a photosensitive surface, all photoelectrons do not come out with the same energy, because in addition to the work done to free electrons from the surface, different (emitted) photoelectrons need different amount of work to be done on them to reach the surface.
Question 51.
A photon and a proton have the same de-Broglie wavelength. Show, by actual calculations, which has more total energy. (Comptt. All India 2017)
Answer: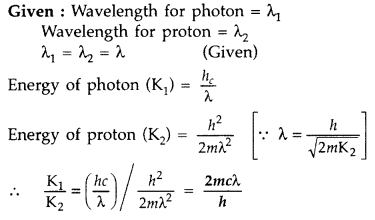
Dual Nature of Radiation and Matter Class 12 Important Questions Short Answer Type SA-II
Question 52.
An electromagnetic wave of wavelength X is incident on a photosensitive surface of negligible work function. If the photoelectrons emitted from this surface have the de-Broglie wavelength λ1, prove that
Answer:
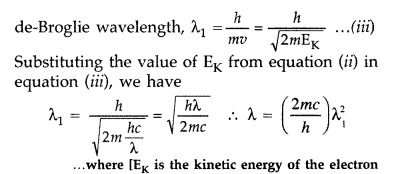
Question 53.
The following graph shows the variation of stopping potential V0 with the frequency v of the incident radiation for two photosensitive metals X and Y :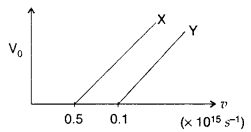
(i) Which of the metals has larger threshold wavelength? Give reason.
(ii) Explain, giving reason, which metal gives out electrons, having larger kinetic energy, for the same wavelength of the incident radiation.
(iii) If the distance between the light source and metal X is halved, how will the kinetic energy of electrons emitted from it change? Give reason. (All India 2008)
Answer: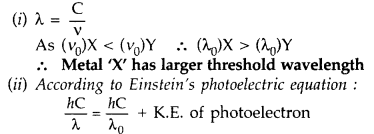
For the same λ of incident radiation, L.H.S. is constant. So metal X with higher value of λ0 will emit photoelectrons of larger K.E.
(iii) Kinetic energy will not change. On reducing the distance only intensity of light changes, frequency remains same. K.E. of emitted photoelectrons depends on frequency.
Question 54.
A proton and an alpha particle are accelerated through the same potential. Which one of the two has
(i) greater value of de-Broglie wavelength associated with it, and
(ii) less kinetic energy? Justify your answers. (Delhi 2008)
Answer: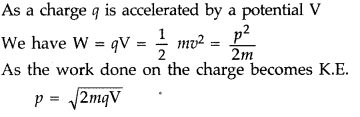
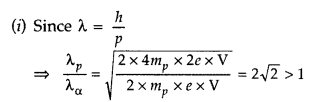
∴ Proton will have greater de-Broglie wavelength
(ii) Energy E = qV. So one proton having lesser charge in coulomb will have less K.E.
Question 55.
An electron and a proton are accelerated through the same potential. Which one of the two has
(i) greater value of de-Broglie wavelength associated with it and
(ii) less momentum? Justify your answer. (Delhi 2008)
Answer: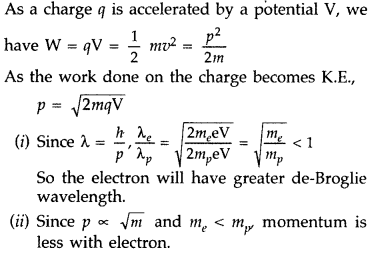
Question 56.
An electron and a photon each have a wavelength of 1.50 nm. Find
(i) their momenta,
(ii) the energy of the photon and
(iii) kinetic energy of the electron. (Delhi 2011)
Answer:
Question 57.
Draw a plot showing the variation of photoelectric current with collector plate potential for two different frequencies, v1 > v2, of incident radiation having the same intensity. In which case will the stopping potential be higher? Justify your answer. (All India 2011)
Answer:
Stopping potential is directly proportional to the frequency of incident radiation. The stopping potential is more negative for higher frequencies of incident radiation. Therefore, stopping potential is higher in v1.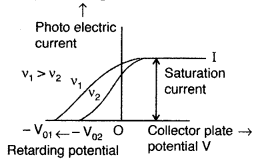
Question 58.
(a) Using de-Broglie’s hypothesis, explain with the help of a suitable diagram, Bohr’s second postulate of quantization of energy levels in a hydrogen atom.
(b) The ground state energy of hydrogen atom is -13.6 eV. What are the kinetic and potential energies of the state?
Answer: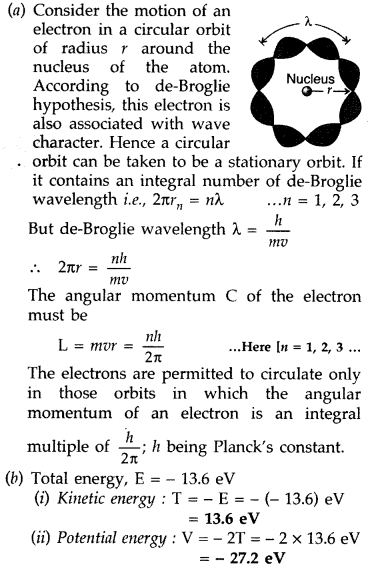
Question 59.
Write Einsten’s photoelectric equation. State clearly how this equation is obtained using the photon picture of electromagnetic radiation. Write the three salient features observed in photoelectric effect which can be explained using this equation. (Delhi 2011)
Answer:![]()
This is Einstein’s photoelectric equation. Photoelectric emission is the result of interaction of two particles—one a photon of incident radiation and other an electron of photo sensitive metal. The free electrons are bound within the metal due to restraining forces on the surface. The minimum energy required to liberate an electron from the metal surface is called work function ϕ0 of the metal. Each photon interacts with one electron. The energy hv of the incident photon is used up in two parts:
(a) a part of the energy of the photon is used in liberating the electron from the metal surface, which is equal to the work function ϕ0 of the metal and
(b) the remaining energy of the photon is used in imparting K.E. of the ejected electron.
By the conservation of energy Energy of the inefficient photon = maximum K.E. of photoelectron + Work function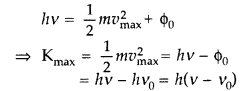
Three salient features are :
Three salient features observed in photoelectric effect on the basis of Einstein’s Photoelectric equation :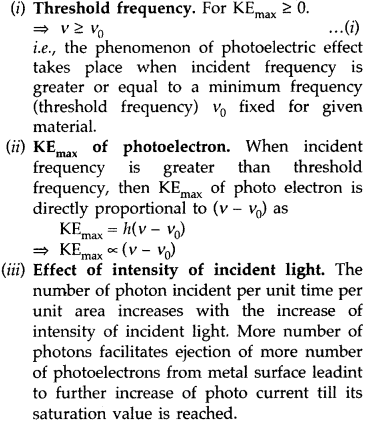
Question 60.
Define the terms
(i) ‘cut-off voltage’ and
(ii) ‘threshold frequency’ in relation to the pheno-menon of photoelectric effect.
Using Einstein’s photoelectric equation show how the cut-off voltage and threshold frequency for a
given photosensitive material can be determined with the help of a suitable plot/graph. (All India 2011) Answer:
(i) Cut-off voltage : The value of the retarding potential at which the photo electric current becomes zero is called cut-off or stopping potential for the given frequency of the incident radiation.
(ii) Threshold frequency : The minimum value of the frequency of incident radiation below which the photoelectric emission stops altogether is called threshold frequency.
According to Eisntein’s photo electric equation,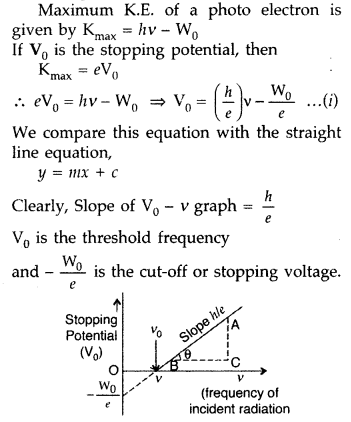
Question 61.
Draw a graph showing the variation of stopping potential with frequency of incident radiation for two photosensitive materials having work functions W1 and W2 (W1 > W2).
Write two important conclusions that can be drawn from the study of these plots. (Comptt. All India 2011)
Answer: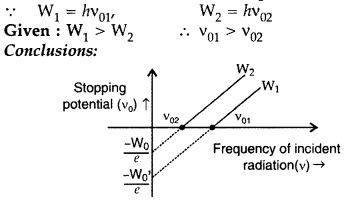
(i) Threshold frequency of material having work function W1 is more than that of material of work function W2.
(ii) The slopes of the straight line graphs, in both the cases, have the same value.
(iii) For the same frequency of incident radiation (> v01), the maximum kinetic energy of the electrons, emitted from the material of work function W1 is < that of electrons emitted from material of work function W2.(any two)
Question 62.
(a) Why photoelectric effect can not be explained on the basis of wave nature of light? Give reasons.
(b) Write the basic features of photon picture of electromagnetic radiation on which Einstein’s photoelectric equation is based. (Delhi 2013)
Answer:
(a) (i) The maximum kinetic energy of the emitted electron should be directly proportional to the intensity of incident radiations but it is not observed experimentally. Also maximum kinetic energy of the emitted electrons should not depend upon incident frequency according to wave theory, but it is not so.
(ii) According to wave theory, threshold frequency should not exist. Light of all frequencies should emit electrons provided intensity of light is sufficient for electrons to eject.
(iii) According to wave theory, photoelectric effect should not be instantaneous. Energy of wave cannot be transferred to a particular electron but will be distributed to all the electrons present in the illuminated portion. Hence, there has to be a time lag between incidence of radiation and emission of electrons.
(b) Basic features of photon picture of electromagnetic radiation :
(i) Radiation behaves as if it is made of particles like photons. Each photon has energy E = hv and momentum p = h/λ.
(ii) Intensity of radiation can be understood in terms of number of photons falling per second on the surface. Photon energy depends only on frequency and is independent of intensity.
(iii) Photoelectric effect can be understood as the result of one to one collision between an electron and a photon.
(iv) When a photon of frequency
(v) is incident on a metal surface, a part of its energy is used in overcoming the work function and other part is used in imparting kinetic energy, so KE = h(v – v0).
Question 63.
Write Einstein’s photoelectric equation and point out any two characteristic properties of photons on which this equation is based. Briefly explain the three observed features which can be explained by this equation. (All India, Comptt. All India 2013)
Answer: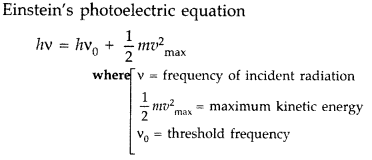
Two characteristics properties of photons on which equation is based.
1. Photoelectric emission will take place only if frequency of incident radiation is greater than or equal to threshold frequency.
2. When a photon collides with an electron, it gives all its energy to electron.
Three features :
Question 64.
(a) State three important properties of photons which describe the particle picture of electromagnetic radiation.
(b) Use Einstein’s photoelectric equation to define the terms
(i) stopping potential and
(ii) threshold frequency. (Comptt. Delhi 2013)
Answer:
(a)
Basic features of photon picture of electromagnetic radiation :
(i) Radiation behaves as if it is made of particles like photons. Each photon has energy E = hv and momentum p = h/λ.
(ii) Intensity of radiation can be understood in terms of number of photons falling per second on the surface. Photon energy depends only on frequency and is independent of intensity.
(iii) Photoelectric effect can be understood as the result of one to one collision between an electron and a photon.
(iv) When a photon of frequency
(v) is incident on a metal surface, a part of its energy is used in overcoming the work function and other part is used in imparting kinetic energy, so KE = h(v – v0).
(b) (i) Stopping potential or cut-off potential. The minimum value of the negative potential ‘V0‘, which should be applied to the anode in a photo cell so that the photo electric current becomes zero, is called stopping potential.
The maximum kinetic energy (Kmax) of photoelectrons is given by,![]()
(ii) Threshold frequency. The minimum frequency V0, which the incident light must possess so as to eject photoelectrons from a metal surface, is called threshold frequency of the metal.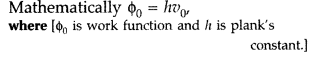
Question 65.
An electron microscope uses electrons accelerated by a voltage of 50 kV. Determine the de-Broglie wavelength associated with the electrons. Taking other factors, such as numerical aperture etc. to be same, how does the resolving power of an electron microscope compare with that of an optical microscope which uses yellow light? (All India 2014)
Answer: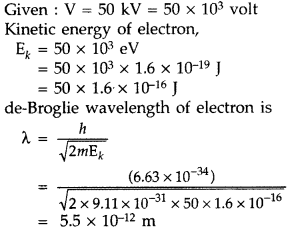
For yellow light, wavelength X = 5.9 × 10-7 m Since resolving power (R.P.) is inversely proportional to wavelength, therefore, R.P. of an electron microscope is about 105 times more than optical microscope.
Question 66.
Write Einstein’s photoelectric equation and mention which important features in photoelectric effect can be explained with the help of this equation.
The maximum kinetic energy of the photoelectrons gets doubled when the wavelength of light incident on the surface changes from λ1 to λ2. Derive the expressions for the threshold wavelength λ0 and work function for the metal surface. (Delhi 2014)
Answer: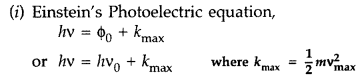
(ii) Important features of photoelectric effect:
(a) Radiation behaves as if it is made of particles like photons. Each photon has energy E = hv and momentum p = h/λ.
(b) Intensity of radiation can be understood in terms of number of photons falling per second on the surface. Photon energy
depends only on frequency and is independent of intensity.
(c) Photoelectric effect can be understood as the result of the one to one collision between an electron and a photon.
(d) When a photon of frequency
(v) is incident on a metal surface, a part of its energy is used in overcoming the work function and other part is used in imparting kinetic energy, so KE = h(v – v0)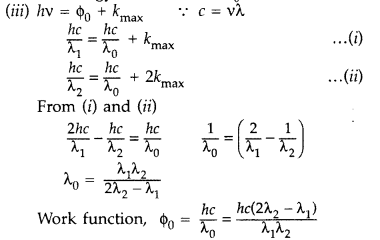
Question 67.
(a) Describe briefly three experimentally observed features in the phenomenon of photoelectric effect.
(b) Discuss briefly how wave theory of light cannot explain these features. (All India 2014)
Answer:
(a) Experimental features and observations of photoelectric effect :
(i) For a given photosensitive material and frequency of incident radiation (above the threshold frequency), the photoelectric current is directly proportional to the intensity of incident light.
(ii) For a given photosensitive material and frequency of incident radiation, saturation current is found to be proportional to the intensity of incident radiation whereas the stopping potential is independent of its intensity.
(iii) For a given photosensitive material, there exists a certain minimum cut-off frequency of the incident radiation, called the threshold frequency, below which no emission of photoelectrons takes place, no matter how intense the incident light is. Above the threshold frequency, the stopping potential or equivalently the maximum kinetic energy of the emitted photoelectrons increases linearly with the frequency of the incident radiation, but is independent of its intensity.
(iv) The photoelectric emission is an instantaneous process without any apparent time lag (~10-9 s or less), even when the incident radiation is made exceedingly dim.
(b) Wave theory cannot explain photoelectric effect:
(i) According to the wave picture of light, the free electrons at the surface of the metal (over which the beam of radiation falls) absorb the radiant energy continuously. The greater the intensity of radiation, the greater are the amplitude of electric and magnetic fields. Consequently, the greater the intensity, the greater should be the energy absorbed by each electron. In this picture, the maximum kinetic energy of the photoelectrons on the surface is then expected to increase with increase in intensity. Also, no matter what the ‘ frequency of radiation is, a sufficiently intense beam of radiation (over sufficient time) should be able to impart enough energy to the electrons, so that they exceed the minimum energy needed to escape from the metal surface. A threshold frequency, therefore, should not exist. These expectations of the wave theory directly contradict observations (a) (i), (ii) and (iii) given above.
(ii) In the wave picture, the absorption of energy by electrons takes place continuously over the entire wavefront of the radiation. Since a large number of electrons absorb energy, the energy absorbed per electron per unit time turns out to be small. Explicit calculations estimate that it can take hours or more for a single electron to pick up sufficient energy to overcome the work function and come out of the metal. This conclusion is again in striking contrast to observation (iv) that the photoelectric emission is instantaneous.
In short, the wave picture is unable to explain the most basic features of photoelectric emission.
Question 68.
(a) Write the important properties of photons which are used to establish Einstein’s photoelectric equation.
(b) Use this equation to explain the concept of
(i) threshold frequency and
(ii) stopping potential. (All India 2014)
Answer:
(a) Important properties of Photons :
(i) In interaction of radiation with matter, radiation behaves as if it is made up of particles called photons.
(ii) Each photon has energy E (= hv) and momentum p (= hv/c), and speed c, the speed of light.
(iii) All photons of light of a particular frequency v, or wavelength λ, have the same energy E (= hv = hc/λ) and momentum p (= hv/c = h/λ), whatever the intensity of radiation may be. By increasing the intensity of light of given wavelength, there is only an increase in the number of photons per second crossing a given area, with each photon having the same energy. Thus, photon energy is independent of intensity of radiation.
(iv) Photons are electrically neutral and are not deflected by electric and magnetic fields.
(v) In a photon-particle collision (such as photon-electron collision), the total energy and total momentum are conserved. However, the number of photons may not be conserved in a collision. The photon may be absorbed or a new photon may be created.
(b) Einstein’s photoelectric equation is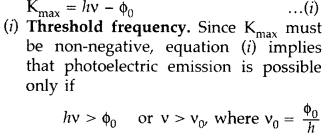
This equation shows that the greater the work function ϕ0, higher the threshold frequency v0 needed to emit photoelectrons.
Thus, there exists a threshold frequency v0 (=ϕ0/h the metal surface, below which no photoelectric emission is possible, no matter how intense the incident radiation may be or how long it falls on the surface.
(ii) Stopping potential. The minimum value of negative potential v0, which should be applied to the anode in a photocell, so that the photoelectric current becomes zero, is called Stopping potential.![]()
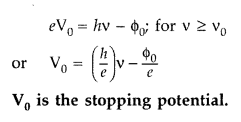
Question 69.
Write three characteristic features in photoelectric effect which cannot be explained on the basis of wave theory of light, but can be explained only using Einstein’s equation. (Delhi 2016)
Answer:
(a) (i) The maximum kinetic energy of the emitted electron should be directly proportional to the intensity of incident radiations but it is not observed experimentally. Also maximum kinetic energy of the emitted electrons should not depend upon incident frequency according to wave theory, but it is not so.
(ii) According to wave theory, threshold frequency should not exist. Light of all frequencies should emit electrons provided intensity of light is sufficient for electrons to eject.
(iii) According to wave theory, photoelectric effect should not be instantaneous. Energy of wave cannot be transferred to a particular electron but will be distributed to all the electrons present in the illuminated portion. Hence, there has to be a time lag between incidence of radiation and emission of electrons.
Question 70.
Sketch the graph showing variation of stopping potential with frequency of incident radiations for two photosensitive materials A and B having threshold frequencies vA > vB.
(i) In which case is the stopping potential more and why?
(ii) Does the slope of the graph depend on the nature of the material used? Explain. (All India 2016)
Answer: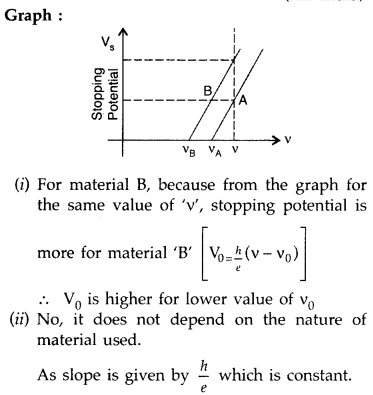
Question 71.
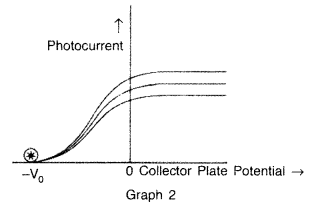
The graphs, drawn here, are for the phenomenon of photoelectric effect.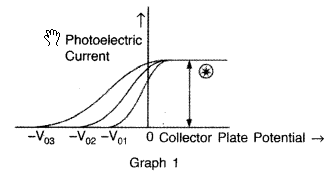
(i) Identify which of the two characteristics (intensity/frequency) of incident light, is being kept constant in each case.![]()
(iii) Justify the existence of a ‘threshold frequency’ for a given photosensitive surface. (Comptt. Delhi 2016)
Answer:
(i) (a) In graph 1 : intensity is being kept constant.
(b) In graph 2 : frequency is being kept constant.
(ii) (a) In graph 1 : Saturation current
(b) In graph 2 : Stopping potential.
(iii) For a given photo-sensitive surface electrons need a minimum energy to be emitted, this is called work function of the surface W.
∴ Photons energy hv should be greater/ equal to the work function.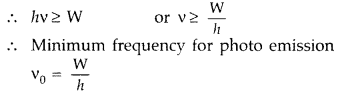
which is justified to be called as threshold frequency.
Question 72.
Point out two distinct features observed experimentally in photoelectric effect which’ cannot be explained on the basis of wave theory of light. State how the ‘photon picture’ of light provides an explanation of these features. (Comptt. All India 2016)
Answer:
(a) (i) The maximum kinetic energy of the emitted electron should be directly proportional to the intensity of incident radiations but it is not observed experimentally. Also maximum kinetic energy of the emitted electrons should not depend upon incident frequency according to wave theory, but it is not so.
(ii) According to wave theory, threshold frequency should not exist. Light of all frequencies should emit electrons provided intensity of light is sufficient for electrons to eject.
(iii) According to wave theory, photoelectric effect should not be instantaneous. Energy of wave cannot be transferred to a particular electron but will be distributed to all the electrons present in the illuminated portion. Hence, there has to be a time lag between incidence of radiation and emission of electrons.
(b) Basic features of photon picture of electromagnetic radiation :
(i) Radiation behaves as if it is made of particles like photons. Each photon has energy E = hv and momentum p = h/λ.
(ii) Intensity of radiation can be understood in terms of number of photons falling per second on the surface. Photon energy depends only on frequency and is independent of intensity.
(iii) Photoelectric effect can be understood as the result of one to one collision between an electron and a photon.
(iv) When a photon of frequency
(v) is incident on a metal surface, a part of its energy is used in overcoming the work function and other part is used in imparting kinetic energy, so KE = h(v – v0).
Question 73.
(i) How does one explain the emission of electrons from a photosensitive surface with the help of Einstein’s photoelectric equation?
(ii) The work function of the following metals is given : Na = 2.75 eV, K = 2.3 eV, Mo = 4.17 eV and Ni 5.15 eV. Which of these metals will not cause photoelectric emission for radiation of wavelength 3300 A from a laser source placed 1 m away from these metals? What happens if the laser source is brought nearer and placed 50 cm away? (Delhi 2017)
Answer: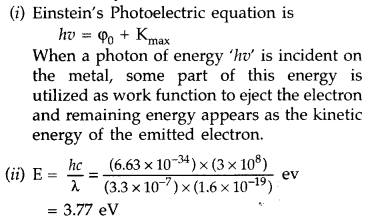
Because the work function of Mo and Ni is more than the energy of the incident photons; so photoelectric emission will not take place from these two metals Mo and Ni. When the laser source is brought nearer and placed 50 cm away, the kinetic energy of photo-electrons will not change, only photoelectric current will change.
Question 74.
In the study of a photoelectric effect the graph between the stopping potential V and frequency v of the incident radiation on two different metals P and Q is shown here:
(i) Which one of the two metals has higher threshold frequency?
(ii) Determine the work function of the metal which has greater value.
(iii) Find the maximum kinetic energy of electron emitted by light of frequency 8 × 1014 Hz for this metal. (Delhi 2017)
Answer: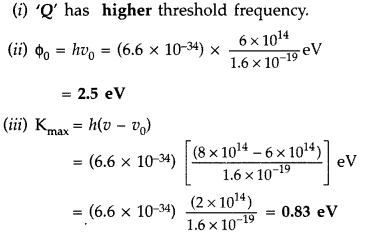
Question 75.
(i) State two important features of Einstein’s photoelectric equation.
(ii) Radiation of frequency 1015 Hz is incident on two photosensitive surfaces P and Q. There is no photoemission from surface P. Photoemission occurs from surface Q but photoelectrons have zero kinetic energy. Explain these observations and find the value of work function for surface Q. (Delhi 2017)
Answer:
(i)
Important features of photoelectric effect:
(a) Radiation behaves as if it is made of particles like photons. Each photon has energy E = hv and momentum p = h/λ.
(b) Intensity of radiation can be understood in terms of number of photons falling per second on the surface. Photon energy
depends only on frequency and is independent of intensity.
(c) Photoelectric effect can be understood as the result of the one to one collision between an electron and a photon.
(d) When a photon of frequency
(v) is incident on a metal surface, a part of its energy is used in overcoming the work function and other part is used in imparting kinetic energy, so KE = h(v – v0)
(ii) Since no photoelectric emission takes place from P, it means frequency of incident radiation (1015 Hz) is less than its threshold frequency (v0)p.
Photo emission takes place from Q but kinetic energy of photoelectrons is zero. This implies that frequency of incident radiation is just equal to the threshold frequency of Q.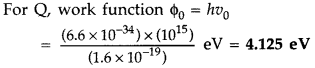
Question 76.
Using photon picture of light, show how Einstein’s photoelectric equation can be established. Write two features of photoelectric effect which cannot be explained by wave theory. (All India 2017)
Answer:
1st part :![]()
This is Einstein’s photoelectric equation. Photoelectric emission is the result of interaction of two particles—one a photon of incident radiation and other an electron of photo sensitive metal. The free electrons are bound within the metal due to restraining forces on the surface. The minimum energy required to liberate an electron from the metal surface is called work function ϕ0 of the metal. Each photon interacts with one electron. The energy hv of the incident photon is used up in two parts:
(a) a part of the energy of the photon is used in liberating the electron from the metal surface, which is equal to the work function ϕ0 of the metal and
(b) the remaining energy of the photon is used in imparting K.E. of the ejected electron.
By the conservation of energy Energy of the inefficient photon = maximum K.E. of photoelectron + Work function
Three salient features are :
Three salient features observed in photoelectric effect on the basis of Einstein’s Photoelectric equation :
2nd part :
(a) (i) The maximum kinetic energy of the emitted electron should be directly proportional to the intensity of incident radiations but it is not observed experimentally. Also maximum kinetic energy of the emitted electrons should not depend upon incident frequency according to wave theory, but it is not so.
(ii) According to wave theory, threshold frequency should not exist. Light of all frequencies should emit electrons provided intensity of light is sufficient for electrons to eject.
(iii) According to wave theory, photoelectric effect should not be instantaneous. Energy of wave cannot be transferred to a particular electron but will be distributed to all the electrons present in the illuminated portion. Hence, there has to be a time lag between incidence of radiation and emission of electrons.
Question 77.
Explain giving reasons for the following:
(a) Photoelectric current in a photocell increases with the increase in the intensity of the incident radiation.
(b) The stopping potential (V0) varies linearly with the frequency (v) of the incident radiation for a given photosensitive surface with the slope remaining the same for different surfaces.
(c) Maximum kinetic energy of the photoelectrons is independent of the intensity of incident radiation. (All India 2017)
Answer:
(a) The collision of a photon can cause emission of a photoelectron (above the threshold frequency). As the intensity increases, number of photons increases. Hence, the current increases.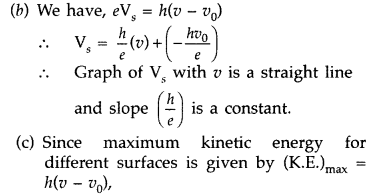
hence, it depends on the frequency and not on the intensity of the incident radiation.
Question 78.
The given graph shows the variation of photocurrent for a photosensitive metal: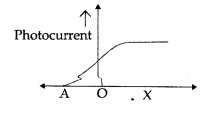
(a) Identify the variable X on the horizontal axis.
(b) What does the point A on the horizontal axis represent?
(c) Draw this graph for three different values of frequencies of incident radiation v1 v2 and v3 (v1 > v2 > v3) for same intensity.
(d) Draw this graph for three different values of intensities of incident radiation I1 I2 and I3 (I1 > I2 > I3) having same frequency. (All India 2017)
Answer:
(a) ‘X’ is a collector plate potential.
(b) ‘A’ represents the stopping potential.
(c) Graph for different frequencies :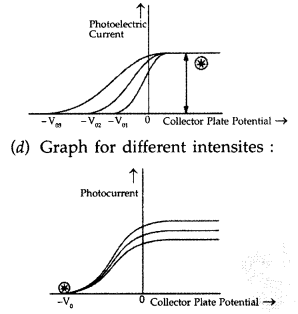
Question 79.
Draw a graph showing the variation of de Broglie wavelength of a particle of charge q and mass m with the accelerating potential. Proton and deuteron have the same de Broglie wavelengths. Explain which has more kinetic energy. (Comptt. Delhi 2017)
Answer: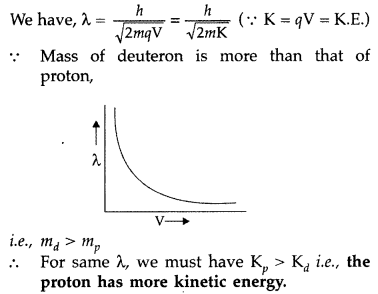
Question 80.
(a) Draw the graph showing the variation of de Broglie wavelength of a particle of charge q and mass m with the accelerating potential.
(b) An electron and proton have the same de Broglie wavelengths. Explain, which of the two has more kinetic energy. (Comptt. Delhi 2017)
Answer:
(a) For Graph :
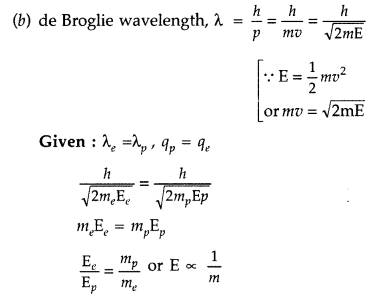
Since the mass of electron is less than that of proton, hence electron will have more kinetic energy.
Question 81.
Draw a graph showing the variation of de Broglie wavelength λ of a particle of charge q and mass m, with the accelerating potential V. An α-particle and a proton have the same de-Broglie wavelength equal to 1Å. Explain with calculations, which of the two has more kinetic energy. (Comptt. Delhi 2017)
Answer:
For Graph
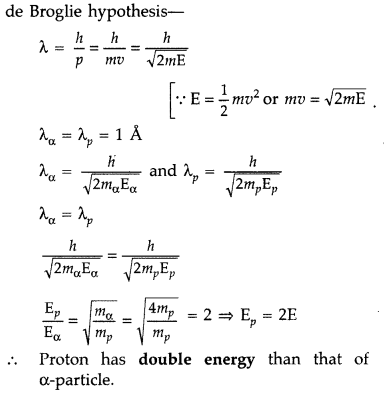
Question 82.
The photon emitted during the de-excitation from the 1st excited level to the ground state of hydrogen atom is used to irradiate a photo cathode of a photocell, in which stopping potential of 5 V is used. Calculate the work function of the cathode used. (Comptt. All India 2017)
Answer: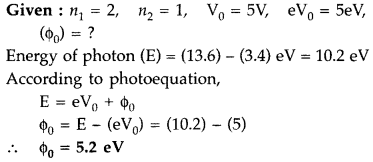
Question 83.
An electron microscope uses electrons accelerated by a potential difference 50 kV. Calculate the de Broglie wavelength of the electrons. Compare the resolving power of an electron microscope with that of an optical microscope, which uses visible light of wavelength 550 nm. Assume the numerical aperture of the objective lens of both microscopes are the same. (Comptt. All India 2017)
Answer:
Dual Nature of Radiation and Matter Class 12 Important Questions Long Answer Type
Question 84.
(a) Write three observed features of photoelectric effect which cannot be explained by wave theory of light.
Explain how Einstein’s photoelectric equation is used to describe these features satisfactorily.
(b) Figure shows a plot of stopping potential (v0) with frequency (v) of incident radiation for two photosensitive materials M1 and M2.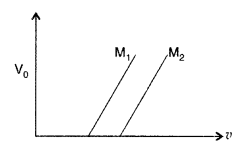
Explain
(i) why the slope of both the lines is same?
(ii) for which material emitted electrons have greater kinetic energy for the same frequency of incident radiation? (Comptt. All India 2017)
Answer:
(a)
(i) The maximum kinetic energy of the emitted electron should be directly proportional to the intensity of incident radiations but it is not observed experimentally. Also maximum kinetic energy of the emitted electrons should not depend upon incident frequency according to wave theory, but it is not so.
(ii) According to wave theory, threshold frequency should not exist. Light of all frequencies should emit electrons provided intensity of light is sufficient for electrons to eject.
(iii) According to wave theory, photoelectric effect should not be instantaneous. Energy of wave cannot be transferred to a particular electron but will be distributed to all the electrons present in the illuminated portion. Hence, there has to be a time lag between incidence of radiation and emission of electrons.
(b)
(i) The slope (V0/v) of both the lines is the same and represents the universal constant known as ‘Planck’s constant’ (h) = 6.62 × 10-34JS
(ii) For the same frequency of incident radiations, M1 will have greater kinetic energy, because the value of V0 is greater for M1 material. It can be easily seen by drawing a vertical line (frequency being the same) and intersecting M1 and M2 at different points (V0 for M1 is higher)
Question 85.
(a) Describe briefly how wave nature of moving electrons was established experimentally.
(b) Estimate the ratio of de-Broglie wavelength associated with deuterons and a-particles when they are accelerated from rest through the same accelerating potential V. (Comptt. All India 2017)
Answer:
(a) Davisson and Germer experiment for wave nature of moving electrons : The experimental arrangement used by Davisson and Germer is schematically shown in the fig. It consists of an electron gun which comprises of . a tungsten filament F, coated with barium oxide and heated by a low voltage power supply (L.T. or battery). Electrons emitted by the filament are accelerated to a desired velocity by applying suitable potential/voltage from a high voltage power supply (H.T. or battery). They are made to pass through a cylinder with fine holes along its axis, producing a fine collimated beam.
The beam is made to fall on the surface of a nickel crystal. The electrons are scattered in all directions by the atoms of the crystal. The intensity of the electron beam, scattered in a given direction, is measured by the electron detector (collector)/ The detector can be moved on a circular scale and is connected to a sensitive galvanometer, which records the current. The deflection of the galvanometer is proportional to the intensity of the electron beam entering the collector. The apparatus is enclosed in an evacuated chamber. By moving the detector on the circular scale at different positions, the intensity of the scattered electron beam is measured for different values of angle of scattering 0 which is the angle between the incident and the scattered electron beams. The variation of the intensity (I) of the scattered electrons with the angle of scattering θ is obtained for different accelerating voltages.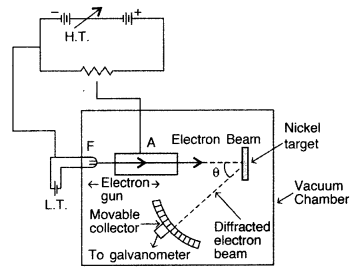
The experiment was performed by varying the accelarating voltage from 44 V to 68 V. It was noticed that a strong peak appeared in the intensity (I) of the scattered electron for an accelarating voltage of 54V at a scattering angle θ = 50°.
The appearance of the peak in a particular direction is due to the constructive interference of electrons scattered from different layers of the regularly spaced atoms of the crystals. From the electron diffraction measurements, the wavelength of matter waves was found to be 0.165 nm.
The de Broglie wavelength λ. associated with electrons, is given by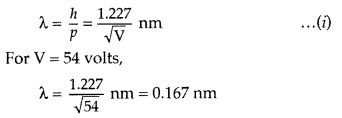
Thus, there is an excellent agreement between the theoretical value and the experimentally obtained value of de Broglie wavelength. Davisson-Germer experiment thus strikingly confirms the wave nature of electrons and the de Broglie relation.