Classification of Elements and Periodicity in Properties Important Extra Questions Very Short Answer Type
Question 1.
An element is present in the third period of the p-block. It has 5 electrons in its outermost shell. Predict its group. How many unpaired electrons does it have?
Answer:
It belongs to the 15th group (P). It has 3 unpaired electrons.
Question 2.
An element X with Z = 112 has been recently discovered. Predict its electronic configuration and suggest the group in which it is present.
Answer:
[Rn] 5f14 6d10 7s2. It belongs to the 12th group.
Question 3.
The electronic configuration of an element is 1s2 2s2 2p6 3s2 3p5. Name the period and the group to which it belongs?
Answer:
Third-period Group 17.
Question 4.
Arrange Cl, Cl–, Cl+ ion in order of increasing size.
Answer:
CP < Cl < CP.
Question 5.
Arrange the following in increasing order of size.
N3-, Na+, F–, O2-, Mg2+
Answer:
Mg2+< Na+< F– < O2- < N3-
Question 6.
Give the formula of one species positively charged and one negatively charged that will be isoelectronic with Ne.
Answer:
Na+, F–.
Question 7.
Argon has atomic number 18 and belongs to the 3rd period and 18th group. Predict the group and period for the element having atomic number 19.
Answer:
Group I, Period 4th.
Question 8.
Which of these belong to
(i) the same period and
(ii) the same group.
| Element | Atomic Number |
| A | 2 |
| B | 10 |
| C | 5 |
Answer:
A and B belong to the same group (18th group)
B and C belong to the same period (II period).
Question 9.
Among the elements of the 3rd period, Na to Al pick out the element.
(i) With the highest first ionization enthalpy.
Answer:
Argon
(ii) With the largest atomic radius.
Answer:
Sodium
(iii) That is the most reactive non-metal.
Answer:
Chlorine
(iv) That is the most reactive metal.
Answer:
Sodium.
Question 10.
Arrange the following ions in the order of increasing size:
Be2+, Cl– , S2-, Na+, Mg2+, Br.
Answer:
Be2+ < Mg2+ < Na+ < Cl– < S2- < Br–
Question 11.
Arrange the following elements in the increasing order of metallic character: B, Al, Mg, K.
Answer:
B < Al < Mg < K
Question 12.
Among the elements, Li, K, Ca, S and Kr which one is expected to have the lowest first ionization enthalpy and which one the highest first ionization enthalpy?
Answer:
K has the lowest IE1
Kr has the highest IE1
Question 13.
Predict the position of the element in the periodic table satisfying the electronic configuration (n – 1)d1 ns2 for n = 4.
Answer:
(n – 1 )d1 ns2 for n = 4 becomes 3d1 4s2. It lies in the 4th period and, in the 3rd group.,
Question 14.
Predict the formulae of the stable binary compounds that would be formed by the following pairs of elements:
(a) Silicon and Oxygen
Answer:
SiO2
(b) Aluminium and Bromine
Answer:
AlBr3
(c) Calcium and Iodine
Answer:
CaI2
(d) Element 114 and Fluorine
Answer:
Uuq F4
(e) Element 120 and Oxygen.
Answer
(Ubn) O
Question 15.
Consider the elements N, P, O, and S and arrange them in order of
(a) increasing first ionization enthalpy
Answer:
S < O < P < N
(b) increasing negative electron gain enthalpy
Answer:
N < P < O < S
(c) increasing non-metallic character.
Answer:
P < N < S < O
Question 16.
Among the elements B, Al, C, and Si
(a) Which has the highest IE?
Answer:
C (Carbon)
(b) Which has the highest negative electron gain enthalpy?
Answer:
C (Carbon)
(c) Which has the largest atomic radius?
Answer:
Al
(d) Which has the most metallic character?
Answer:
Al
Question 47.
Arrange the following ions in order of decreasing ionic radii: Li2+, He+, Be3+.
Answer:
He+, Li2+, Be3+ are all isoelectronic ions
∴ He+ > Li2+ > Be3+.
Question 18.
Name the group of elements classified as s-, p-, d-blocks.
Answer:
- s-block consists up of groups 1 and 2. Alkali and alkaline earth metals.
- p-block consists up of groups 13-18.
- d-block consists up of groups 3-12, called transition elements.
Question 19.
Al atom loses electrons successively to form Al+, Al2+, Al3+ ions. Which step will have the highest ionization enthalpy?
Answer:![]()
will have the highest IE.
Question 20.
In terms of electronic configuration, what the elements of a given period and a given group have in common?
Answer:
In a given period, the no. of shells is equal and in a given group, the no. of electrons in the valence shell is the same.
Question 21.
Which of the following elements has the most positive electron gain enthalpy? Fluorine, Nitrogen, Neon.
Answer:
Neon.
Question 22.
Why electron affinities of Be and Mg are positive?
Answer:
They have fully s-orbitals and the additional electron cannot be placed in the much higher energy p-orbitals of the valence shell.
Question 23.
What would be the IUPAC name and symbol for the element with atomic number 120?
Answer:
Unbinillium and its symbol are Ubn.
Question 24.
Why ionization enthalpy of Nitrogen is greater than that of oxygen?
Answer:
Nitrogen has. exactly half-filled orbitals, due to which it is difficult to remove an electron from the valence shell of N atoms.
Question 25.
Give four examples of species that are isoelectronic with Ca2+.
Answer:
Ar, K+, S2-, P3- are isoelectronic with Ca2+.
Question 26.
Comment on the statement, “The electron gain enthalpy of halogens decrease in the order F > Cl > Br > I.”
Answer:
The statement is wrong. The actual order is I > Br > F > Cl.
Question 27.
Arrange the following in order of increasing radii I, I+, I–.
Answer:
I+ < I < I–
Question 28.
Which of the following pairs would have a larger size
(i) K or K+
Answer:
K
(ii) Br or Br
Answer:
Br
(iii) O2- or F–
Answer:
O2-
(iv) Na+ or K+
Answer:
K+
Question 29.
Select from each group the one with the smallest radius.
(i)O, O–, O2-
Answer:
O
(ii) K+, Sr2+, Ar
Answer:
Sr2+
(iii) Si, P, Cl
Answer:
Cl
Question 30.
Which of the following has the largest and smallest size? Mg, Mg2+, Al, Al3+.
Answer:
Mg has the largest and Al3+ the smallest size.
Question 31.
What is the recommended, IUPAC name and symbol of the element with atomic number 108?
Answer:
Element with At. No. = 108 is Uno. Its recommended name is Unniloctium and its official IUPAC .name is Hassnium.
Question 32.
What is the general outer shell electronic configuration of p-block elements?
Answer:
ns2 np1-6 where n = 2 to 6.
Question 33.
What is the general outer shell electronic configuration of s-block elements?
Answer:
ns1 ~ 2, where n = 2 to 7.
Question 34.
Give the general outer shell electronic configuration of d-block elements?
Answer:
(n – 1 )d1-10 ns0-2 where n = 4 to 7.
Question 35.
What is the general outer shell electronic configuration of f-block elements?
Answer:
(n -2)f0-14 (n – l)d0-2 ns2 where n = 6 – 7.
Question 36.
Which are the three elements among the first three transition series (1st, 2nd, and 3rd) which generally do not show v that properties shown by other members of the series.
Answer:
Zn, Cd, and Hg (Zinc, cadmium, and mercury).
Question 37.
Which is the shortest period in the modern periodic table? Name the elements in it.
Answer:
First period. Hydrogen (H) and helium (He).
Question 38.
What is the number of groups in the d-block?
Answer:
Group numbers 3, 4, 5, 6, 7, 8, 9, 10,11, 12 belong to the d- block
Question 39.
Give the electronic configuration of the first and the last element of the I transition series.
Answer:
Sc (21) = 1s2, 2s2 2p6, 3s2 3p6 3d104s2 .
Zn (30) = 1s2, 2s2 2p6, 3s2 3p6 3d10 4s2.
Question 40.
Give the names and symbols of two metalloids.
Answer:
Arsenic (As) and antimony (sb).
Question 41.
What do you mean by Vander Waals’ radius?
Answer:
It is defined as one-half the distance between the nuclei of two identical non-bonded isolated atoms or two adjacent identical atoms belonging to two neighboring molecules of an element in the solid state.
Question 42.
Arrange the following ions in order of decreasing ionic radii: Li2+, He+, Be3+.
Answer:
He+> Li2+> Be3+.
Question 43.
Arrange the following in decreasing, order of their van der Waals radii: Cl, H, O, N.
Answer:
Cl > N > O > H. .
Question 44.
What are super heavy elements?
Answer:
Elements with Z > 100 which have high densities are called superheavy elements.
Question 45.
Which transition element has the maximum oxidation state?
Answer:
Osmium (Os) shows an oxidation state of + 8.
Question 46.
Why do the elements of the 2nd period show anomalous properties than the other members of their respective groups?
Answer:
- small size
- large charge/radius ratio
- high electronegativity
- Absence of d-orbitals.
Question 47.
Which group elements in the periodic table show the maximum electronegativity in their compounds?
Answer:
Halogens belonging to the 17th group.
Question 48.
For each of the following pairs, predict which one has lower first ionization enthalpy?
(i) N or O
Answer:
O
(ii) Na or Na+
Answer:
Na
(iii) Be+ or Mg2+
Answer:
Be+
(iv) I or I–
Answer:
I–
Question 49.
Which of the following electronic configurations of the elements do you expect to have a higher value of ionization enthalpy?
(i) 1s2 2s2 2p3 or 1s2 2s2 2p4
Answer:
1s2 2s2 2p3
(ii) 1s2 or 1s2 2s2 2p6
Answer:
1s2
(iii) 3s2 or 3s2 3p1
Answer:
3s2
Question 50.
Arrange the following elements in order of decreasing electron gain enthalpy: B, C, N O.
Answer:
N > B > C > O.
Classification of Elements and Periodicity in Properties Important Extra Questions Short Answer Type
Question 1.
Do elements with high I.E. have high E.A.?
Answer:
Normally is true that the elements with haying high value of I.E. have a high value of E affinity. But however, there are marked exceptions. It is seen that elements, with stable electronic configurations, have very high values of I-Energies as it is difficult to remove electrons as is the case with 15th and 18th group elements but in such case electron cannot be added easily so that is why elements of 15th group have almost zero E.A. and elements of 18th group have got zero E.A. whereas their Ionisation energy values are very high.
Question 2.
What is a periodic classification of elements?
Answer:
By periodic classification of the elements we mean the arrangement of the elements in such a way that the elements with similar physical and chemical properties are grouped together and for this various scientists made contributions but however the contributions made by Mendeleev are of great significance and he gave a periodic table which called as Mendeleev’s Periodic ‘Table which was older and replaced by the long form of the periodic table.
Question 3.
Distinguish between s and p block elements.
Answer:
They can be distinguished as follows: s block elements:
- They have got the general configuration of the valence shell, ns1-2.
- They are all metals.
- Their compounds are mostly ionic.
- They are generally strong reducing agents.
- They mostly impart characteristic color to the flame.
- They have low ionization energies.
- They show fixed oxidation states,
p block elements:
- The valence shell electronic configuration of p block elements in ns2 p1-6.
- They are mostly non-metals.
- Their compounds are mostly covalent.
- They are generally strong oxidizing agents.
- Mostly they do not impart color to the flame.
- They have got a comparatively higher value of I.E.
- They show variable oxidation states. ,
Question 4.
Explain why ionization enthalpies decrease down a group of the Periodic Table.
Answer:
The decrease in ionization enthalpies down any group is because of the following factors:
- There is an increase in the number of the main energy shells
- moving from one element to another.
- There is also an increase in the magnitude of the screening effect due to the gradual increase in the number of inner electrons.
Question 5.
Why does the first ionization enthalpy increase as we go . from left to right across a given period of the Periodic Table?
Answer:
The value of ionization enthalpy increases with the increase in atomic number across the period.
This is due to the fact that in moving across the period from left to right.,
- Nuclear charge increases regularly by one unit.
- The progressive addition of electrons occurs at the same level.
- Atomic size decreases.
This is due to the gradual increase in nuclear charge and a simultaneous decrease in atomic size the electrons are more and more tightly bound to the nucleus. This results in a gradual increase in ionization energy across the period.
Question 6.
How do atomic radii vary across a period with an atomic number in the periodic table? Explain.
Answer:
Variation of Atomic radii across a period: Atomic radii decrease with the increase in the atomic number in a period. For example, atomic radii decrease from lithium to fluorine in the second period.
In moving from left to right across the period, the nuclear charge increases progressively by one unit but the additional electron goes” to the same principal shell. As a result, the electron cloud is pulled closer to the nucleus by increased effective nuclear charge. This causes a decrease in atomic size.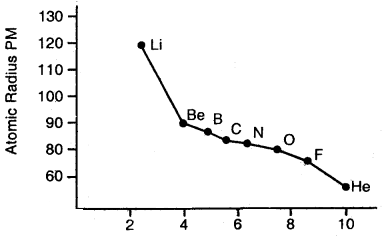
Variation of the atomic radius with an atomic number across the second period
Question 7.
In each of the following pairs, which species has a larger size? Explain.
(i) Kor K+
Answer:
K is larger in size than K+.
The electronic configurations of K and K+ are:
K: 1s2, 2s2 2p6, 3s2 3p6, 4s1
K+: 1s2, 2s2 2p6, 3s2 3p6.
K+ is formed from K when the latter loses its 4s electrons.
K → K+ + e–
As seen from the electronic configurations of K and K+, the K+ ion has 18 electrons and the K atom has 19 electrons but the nuclear charge in both these species is the same (+ 19). Since the K+ ion has 1 electron less than the K atom, the forces of attraction between the nucleus and the electrons are more strong in the K+ ion than in the K atom. This results in more inward pulling of electrons towards the nucleus in K+ ion and hence its size decreases.
(ii) Br or Br
Answer:
Br has formed .by the gain of one electron by Br atom. In Br, the nuclear charge is the same as that in the Br atom but the number of electrons has increased. Since the same nuclear charge how acts on the increased number of electrons, the effective nuclear charge per electron decreases in Br”. The electron cloud is held less tightly by the nucleus. This causes an increase in size.
Question 8.
Explain why chlorine has a higher electron affinity than Fluorine? :
Answer:
Cl has a higher electron affinity than F. This is due to the small size of fluorine. As a result of its very small size the inter-electronic repulsions in relatively compact 2p-subshell of a chlorine atom. On comparing chloride ion with fluoride ion we find that electron density per unit volume in fluoride ion (F–) is more than in chloride (Cl–) ion. This means that the coming election in the fluorine atom finds less attraction than in the chlorine atom. Consequently, the electron affinity of chlorine is higher than that of fluorine.
Question 9.
Two elements C and D have atomic numbers 36 and 58 respectively. On the basis of electronic configuration predict the following:
(i) The group, period, and block to which each element belongs,
Answer:
The electronic configuration of elements C and D are:
C (At. no 36) = 1s2, 2s2 2p6, 3s2 3p6 3d10, 4s2 4p6.
D (At. no. 58) = 1s2, 2s2 2p6, 3s2 3p6 3d10, 4s2 4p6, 5s2 4d10 5p6,6s2 5d1 4f1.
The element C belongs to the 18th group, lies in the 4th period, and belongs to the p-block of elements. The element D belongs to lanthanides, lies in the 6th period, and belongs to/-block of elements.
(ii) Are they representative elements?
Answer:
The element C is a noble gas, and D is the inner transition element. They are not representative elements.
Question 10.
Account for the fact that the fourth period has 18 and not 8 elements.
Answer:
The first element of fourth period is potassium (Z = 19) having electronic configuration 1s2 2s2 2p6 3s2 3p6 4s1. Thus it starts with the filling of 4s orbital., After the filling of 4s orbital, instead of 4p orbitals, there start the filling 3d orbitals. This is in keeping with the fact that 3d orbitals have fewer energies than 4p orbitals.
Thus 10 elements are built up by the filling of 3d orbitals. After the filling of 3d orbitals, 4p orbitals are filled up and that process is completed at krypton [Kr, Z = 36]. Hence the fourth period consists up of 18 and not 8 elements.
Question 11.
Explain the term ‘valency’ of an element. How does it vary in a period and in a group in the periodic table?
Answer:
The chemical properties of elements depend upon the number of electrons in the outermost shell of an atom. These electrons are called valence electrons and thus determines the valency of the atom (or element).
In representative elements, the valency is generally equal to either n or (8 – n), where n is no. of. valence electrons in the atom.
In a period valence electrons increase from 0 to 8 on moving from left to light. The valency of an element w.r.t. H and Cl increase from 1 to 4 and then decrease to zero. However w.r.t. oxygen, valency increases from 1 to 7 and then becomes zero in noble gases.
In a group, the number of valence electrons remains the same, and therefore all elements in a group exhibit the same valency, e.g., all elements of group I have valency one and those of group 2 have valency two. However, the transition elements exhibit variable valency.
Question 12.
The elements, Z = 107 and Z = 109 have been made recently, element Z = 108 has not yet been made. Indicate the groups in which you will place the above elements.
Answer:
The element with Z = 107, will be placed in the 7th Group, an element with Z = 108 in group 8th and the element with Z = 109 will be placed in group 9th of the periodic table. All three elements will belong to the d-block of the periodic table. (The valence shell electronic configuration will be 7s2 5f14 6d5 to 7s25f14 6d7).
Question 13.
Lanthanides and actinides are placed in separate rows at the bottom of the periodic table. Explain the reason for this arrangement.
Answer:
Lanthanides and actinides are placed in separate rows at the bottom of the periodic table
- to save space
- to keep elements? with similar properties in a single column and
- because their antepenultimate 4/and 5/orbitals are filled respectively.
Question 14.
Which of the following species will have the largest and the smallest size? Mg, Mg2+, Al, Al3+.
Answer:
Atomic radii decrease from left to right across a period. Cations are always smaller than their parent atoms. Among the isoelectronic species, the one with a larger positive nuclear charge will have a smaller ionic radius.
Hence the largest species is Mg, the smallest one is Al3+
Question 15.
The first ionization enthalpy (IE) of the third-period elements Na, Mg, and Si are respectively 496, 737, and 786 kJ mol-1. Predict whether the first IE value of Al will be more close to 575 or 760 kJ mol-1 Justify your answer.
Answer:
The first ionization enthalpy (IE) of Al will be more close to 575 kJ mol-1. The value of Al should be lower than that of Mg because of the effective shielding of 3p electrons from the nucleus by 3s electrons.
Question 16.
Which of the following will have the most negative electron gain enthalpy and which the least?
P, S, Cl, F. Explain your answer.
Answer:
Electron gain enthalpy generally becomes more negative across a period as we move from left to right. Within a group, electron gain enthalpy becomes less negative down a group. However, adding to electron to 2p orbital leads to greater repulsion than adding an electron to the larger 3p orbital. Hence the element with the most negative electron gain enthalpy is chlorine; the one with the least negative electron gain enthalpy is phosphorus.
Question 17.
Predict the formulae Of compounds which right be formed by the following pairs of elements
(a) Silicon and bromine
Answer:
Silicon (Si) is a group 14 element with a valence of 4; bromine belongs to the group 17 halogen family with a valence of one. Hence the formula of the compound will be SiBr4.
(b) aluminum and sulfur.
Answer:
Aluminum belongs to group 13 with a valence of 3, sulfur belongs to group 16 with a valence of 2. Hence the formula of the compound would be Al2S3.
Question 18.
Show by a chemical reaction with water that Na2O is a basic oxide and C12O7 is an acidic oxide.
Answer:
Na2O reacts with water to form a strong base whereas C12O7 forms a strong acid.
Na2O + H2O → 2NaOH (strong base)
C12O7 + H2O → 2HClO4 (strong acid) ,
Their basic or acidic nature can be qualitatively tested with litmus paper.
Question 19.
Describe the nature of oxides formed by the extreme left elements of the s-blocks and elements on the extreme right Of the p- block.
Answer:
Elements on two extremes of a period easily combine with oxygen to form oxides. The normal oxide formed by the element in the extreme left is the most basic (e.g., Na2O) whereas that formed by the element on the extreme right is the most acidic (e.g., C12O7). Oxides of elements in the center are amphoteric (e.g., Al2O3 As2O3) or neutral (e.g., CO, NO. N2O).
Question 20.
What are super heavy elements?
Answer:
Elements that have Z > 100 and have high densities are called superheavy elements.
Question 21.
Calculate the energy required to convert all atoms of magnesium to magnesium ions present in 24 mg of magnesium vapors. IE1 and IE2 of Mg are 737.76 and 1450.73 kJ mol-1 respectively.
Answer:
Mg (g) + IE1 → Mg+ (g) + e– (g)
IE1 = 737.76 kJ mol-1
Mg+ (g) + IE2 → Mg2+ + e– (g)
IE2 = 1450.73 kJ mol-1
Total amount of energy needed to convert Mg (g) into ,
Mg2+ ion = IE1 + IE2
Now 24 mg of Mg =
= 10-3 mole.
Question 22.
The IE1 and IE2 of Mg. (g) are 740 and 1450 kJ mol-1. Calculate the percentage of Mg+ (g) and Mg2+ (g) if 1 g of Mg (g)
absorbs 50 kJ of energy.
Answer:
No. of moies of Mg vapours present in I g =
Energy absorbed to convèrt Mg (g) to Mg+ (g) = 0.0417 × 740
=30.83 kJ
Energy left unused = 50 – 30.83
= 19.17 kJ
Now 19.17 kJ will be used to e Mg+ (g) to Mg2+ (g) .
∴ No. of moles of Mg+ (g) converted to Mg2+ (g) =
%age of Mg+ (g) =
and %age of Mg2+ (g) = 100 – 68.35 = 31.65
Question 23.
Which of the elements Na, Mg, Si, P would have the greatest difference between the first and the second ionization enthalpies. Briefly explain your answer
Answer:
Among Na, Mg, Si, P, Na is an alkali metal. It has only one electron in the valence shell. Therefore, its IE1 is low: However, after the removal of the first electron, it acquires a Neon gas configuration i.e., Na+ (1s2, 2s2 2p6). Therefore its IE2 is expected to be very high. Consequently, the difference in IE1 and IE2 comes to be greatest in the case of Na.
Question 24.
The IE2 of Mg is higher than that of Na. On the other hand, the IE2 of Na is much higher than that of Mg. Explain,
Answer:
The first electron in both cases has to be removed from the 3s orbital, but the nuclear charge of Na is less than that of Mg. After the removal of the first electron from Na, the electronic configuration of Na+ is 1s2, 2s2 2p6, i.e., that of noble gas which is very stable and the removal of the 2nd electron is very difficult. In the case of Mg after the removal of the first electron, the electronic configuration of Mg+ is 1s2, 2s2 2p6 3s. The 2nd electron to be removed is again from 3s orbital which is easier.
Question 25.
The electron gain enthalpy of chlorine is – 349 kJ mol-1 How much energy in kJ is released when 3.55 g of chlorine is converted completely into Cl– ion in the gaseous state.
Answer:
According to the definition of electron gain enthalpy
Cl(g) + e– (g) → Cl– (g) + 349 kJ mol-1
The energy released when 1 mole (= 35.5 g) of chlorine atoms change completely with Cl– (g) = 349 kJ
Question 26.
The amount of energy released when 1 × 1010 atoms of chlorine in vapor state are converted to Cl– ions according to the equation.
Cl (g) + e– → Cl– (g) is 57.86 × 10-10 J
Calculate the electron gain enthalpy of the chlorine atom in terms of kJ mol-1 and eV pet atom.
Answer:
The electron gain enthalpy of chlorine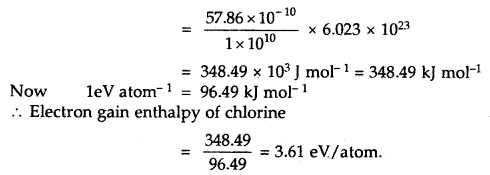
Question 27.
What is electronegativity? How does it vary along a period and within a group?
Answer:
Electronegativity is defined as the tendency/power/urge of an atom in a molecule to attract the shared pair of electrons v towards itself.
- Variation across a period: Electronegativity generally increases from left to right across a period.
- Variation within a group: It generally decreases in going from top to bottom within a group.
Question 28.
Electronic configuration of the four elements are given below:
Arrange these elements in increasing order of their metallic character. Give reasons for your answer.
(i) [Ar]4s2
Answer:
[Ar]4s2 is Calcium metal with At. no. = 20.
(ii) [Ar]3d10 4s2
Answer:
[Ar]3d10 4s2 is Zinc metal with At. no. = 30.
(iii) [Ar]3d10 4s2 4p6 5s2
Answer:
[Ar]3d10 4s2 4p6 5s2 is Strontium metal with At. no. = 38.
(iv) [Arl 3d10 4s2 4p6 5s1
Answer:
[Ar] 3d10 4s2 4p6, 5s1 is*Rubidium metal with At. no. = 37.
Alkali metals are the most metallic, followed by alkaline earth metals and transition metals. Among alkali metals – Rubidium (37) is the most metallic. Among alkaline earth metals (Ca, Sr) Sr (Strontium) is more metallic than Calcium (Ca) as the metallic character increases from top to bottom in a group. Zinc – the transition metal is the least metallic. Thus metallic character increases from
Zn < Ca < Sr < Rb or (ii) < (i) < (iii) < (iv)
Question 29.
lthough F is more electronegative than chlorine but the electron gain enthalpy of Cl is more negative than that of F. Why?
Answer:
L. Pauling gave the highest value of 4.0 to F due to its highest electronegative character. Electronegativity decreases from top to bottom in a group and therefore Cl is less electronegative than F. Electron gain enthalpy of F.is less negative than Cl because of the compact size of its atom (2 orbits) as compared to Cl (3 orbits) the mutual electronic repulsion in F is more than that of Cl.
Question 30.
The formulation of F (g) from F (g) is exothermic whereas that of O2- (g) from O (g) is endothermic. Explain.
Answer:
F (g) + e– (g) → F (g); ΔH = Negative
Energy is released when an extra electron from outside is added to a neutral isolated gaseous atom of an element.
To convert, O (g) to O2- (g) two steps are required
(i) O (g) + e– (g) → O– (g); ΔH1 = – 141 kJ mol-1
(ii) O– (g) + e– (g) → O2- (g); ΔH2 = + 780 kJ mol-1
Hence the over all processes endothermic (+ 780 – 141 = + 639 kJ mol-1) whereas F (g) to F– (g) is exothermic.
Classification of Elements and Periodicity in Properties Important Extra Questions Long Answer Type
Question 1.
Explain the important general characteristics of groups in the modem periodic table in brief.
Answer:
The elements of a group show the following important similar characteristics.
(0 Electronic configuration. All elements in a particular group have similar outer electronic configuration e.g., all elements of group I’, i.e., alkali metals have ns1 configuration in their valency shell. Similarly, group 2 elements (alkaline Earths) haye ns2 outer configuration and halogens (group 17) have ns2 np5 configuration (where n is the outermost shell).
(it) Valency. The valency of an element depends upon the number of electrons in the outermost shell. So elements of a group show the same valency, e.g., elements of group 1 show + 1 valency and group 2 show + 2 valencies i.e. valency i.e., NaCl > MgCl2 etc.
(iii) Chemical properties. The chemical properties of the elements are related to the number of electrons in the outermost shell of their atoms. Hence all elements belonging to the same group show similar chemical properties. But the degree of reactivity varies gradually from top to bottom in a group. For example, in group 1 all the elements are highly reactive metals but the degree of reactivity increases from Li to Cs. Similarly, elements of group 17, i.e., halogens: F, Cl, Br, I are all non-metals and they’re- reactivity goes on decreasing from top to bottom.
Question 2.
Explain the electronic configuration in periods in the periodic table. „
Answer:
Each successive period in the periodic table is associated with the filling Up of the next higher principal energy level (n – 1, n – 2, etc.). It can be readily seen that the number of elements in each period is twice the number of atomic orbitals available in the energy level that is being filled. The first period starts with the filling of the lowest level (1s) and has thus the two elements – hydrogen (1s1) and helium (1s2) when the first shell (K) is completed. The second period starts with lithium and the third electron enters the 2s orbital.
The next element, beryllium has four electrons and has the electronic configuration 1s2 2s2. Starting from the next element boron, the 2p orbitals are filled with electrons when the L shell is completed’ at neon (2s2 2p6). Thus there are 8 elements in the second period. The third period (n = 3) being at sodium, and the added electron enters a 3s orbital. Successive filling of 3s and 3p orbitals give rise to the third period of 8 elements from sodium to argon.
The fourth period (n = 4) starts at potassium with the filling up of 4p of 4s orbital. Before the 4p orbital is filled, the filling up of 3d orbitals becomes energetically favorable and we come across the so-called 3d transition series of elements. The fourth period ends at krypton with the filling up of the 4p orbitals. Altogether we have 18 elements in the fourth period. The fifth period (n = 5) beginning with rubidium is similar to the fourth period and contains the 4d transition series starting at yttrium (Z = 39).
This period ends at xenon with the filling up of the 5p orbitals. The sixth period (n = 6) contains 32 elements and successive electrons enter 6s, 4/, 5d, and 6p orbitals, in that order. Filling up of the 4/ orbitals being with cerium, (Z = 58) and ends at lutetium (Z = 71) to give the 4/-inner transition series which is called the lanthanide series. The seventh period (n = 7) is similar to the sixth period with the successive filling up of the 7s, 5f, 6d, and 7p orbitals and includes most of the man-made radioactive elements.
This period will end at the element with atomic number 118 which would belong to the noble gas family. Filling up of the 5f orbitals after actinium (Z = 89) gives the 5f-inner transition series known as the actinide series. The 4f and 5f transition series of elements are placed separately in the periodic table to maintain its structure and to preserve the principle of classification by keeping elements with similar properties in a single column.
Question 3.
Explain the variation of valence in the periodic table.
Answer:
Variation of valence in a group as well as across a period in the periodic table occurs as follows:
1. In a group: All elements in a group show the same valency. For example, all alkali metals (group 1) show a valency of 1+. Alkaline earth metals (group 2) show a valency of 2+.
However, the heavier elements of p-block elements (except noble gases) show two valences: one equal to the number of valence electrons or 8-No. of valence electron# and the other two less. For example, thallium (Tl) belongs to group 13. It shows valence of 3+ and 1+.
Lead (Pb) belongs to group 14. If shows valance of 4+ and 2+.
Antimony (Sb) and Bismuth (Bi) belong to group 15. They show valence of 5+ and 3+ being more stable.
This happens due to the non-participation of tie two s-electrons present in the valence shell of these elements. This non-participation of one pair of s-electrons in bonding is called the inert-pair effect.
2. In a period: The number of the valence electrons increases – in going from left to right in a period of the periodic table. Therefore the valency of the elements in a period first increases, and then decreases.










