Important Questions of Chemical Reactions and Equations Class 10 Science Chapter 1
Question 1.
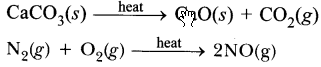
Identify ‘x’, ‘y’ and ‘z’ in the following reaction :![]()
(a) x = gas; y = reaction condition; z = gas
(b) x = solid; y = liquid; z = gas
(c) x = number of moles of KClO3; y = reaction condition; z = number of molecules of oxygen
(d) x = physical state of KClO3 and KCl;
y = reaction condition, z = physical state of O2. (2020)
Answer:![]()
Question 2.
Assertion (A) : Following is a balanced chemical equation for the action of steam on iron : 3Fe + 4H2O → Fe3O4 + 4H2
Reason (R): The law of conservation of mass holds good for a chemical equation.
(a) Both (A) and (R) are true and reason (R) is the correct explanation of the assertion (A)
(b) Both (A) and (R) are true, but reason (R) is not the correct explanation of the assertion (A).
(c) (A) is true, but (R) is false.
(d) (A) is false, but (R) is true. (2020)
Answer:
A balanced chemical equation must obey the law of conservation of mass.
Question 3.
(a) State the law that is followed by balancing a chemical equation.
(b) Balance the following chemical equation: Na + H3O → NaOH + H2 (Board Term I, 2013)
Answer:
(a) Law of conservation of mass is followed for balancing a chemical equation which states that mass can neither be created nor destroyed in a chemical reaction. That is, the total mass of the elements present in the products of a chemical reaction has to be equal to the total mass of the elements present in the reactants in a balanced equation.
(b) 2Na(s) + 2H2O(l) → 2NaOH(aq) + H2(g)
Question 4.
Explain the significance of photosynthesis.
Write the balanced chemical equation involved in the process. (Board Term I, 2017)
Answer:
Photosynthesis means synthesis with the help of light. It is the process that gives life to all living beings.
Photosynthesis is a process by which plants utilize carbon dioxide and water in the presence of sunlight to produce glucose and oxygen.![]()
Question 5.
Write balanced chemical equations for the following chemical reactions:
(a) Hydrogen + Chlorine → Hydrogen chloride
(b) Lead + Copper chloride → Lead chloride + Copper
(c) Zinc oxide + Carbon → Zinc + Carbon monoxide (Board Term I, 2014)
Answer:
(a) H2(g) + Cl2(g) → 2HCl(g)
(b) Pb(s) + CuCl2(aq) → PbCl2(aq)+ Cu(s)
(c) ZnO(s) + C(s) → Zn(s) + CO(g)
Question 6.
Calcium oxide reacts vigorously with water to produce slaked lime.
CaO(s) + H2O(l) → Ca(OH)2(aq)
This reaction can be classified as
(A) Combination reaction
(B) Exothermic reaction
(C) Endothermic reaction
(D) Oxidation reaction
Which of the following is a correct option? (2020)
(a) (A) and (C)
(b) (C) and (D)
(c) (A), (C) and (D)
(d) (A) and (B)
Answer:
(d) The reaction between CaO and H2O to form Ca(OH)2 is an exothermic combination reaction.
Question 7.
When hydrogen sulphide gas is passed through a blue solution of copper sulphate, a black precipitate of copper sulphide is obtained and the sulphuric acid so formed remains in the solution. The reaction is an example of a (2020)
(a) combination reaction
(b) displacement reaction
(c) decomposition reaction
(d) double displacement reaction.
Answer:
(d) CuSO4 + H2S → CuS + H2SO4
It is a double displacement reaction as in this reaction CuSO4 and H2S reacting by exchange of Cu2+ and H+ ions to from two new compounds i.e., CuS and H2SO4.
Question 8.
In a double displacement reaction such as the reaction between sodium sulphate solution and barium chloride solution :
(A) exchange of atoms takes place
(B) exchange of ions takes place
(C) a precipitate is produced
(D) an insoluble salt is produced
The correct option is (2020)
(a) (B) and (D)
(b) (A) and (C)
(c) only (B)
(d) (B), (C) and (D)
Answer:
(d) In this reaction exchange of Na+ and Ba2+ ions takes place forming BaSO4 which is a white precipitate i.e., an insoluble salt.
Na2SO4 + BaCl2 → BaSO4 ↓+ 2NaCl
Question 9.
In which of the following, the identity of initial substance remains unchanged? (2020)
(a) Curdling of milk
(b) Formation of crystals by process of crystallisation
(c) Fermentation of grapes
(d) Digestion of food
Answer:
(b): Formation of crystals is a physical change rest others are chemical change.
Question 10.
Study the following equation of a chemical reaction: (Board Term 1, 2015)
H2 + Cl2 → 2HCl
(i) Identify the type of reaction.
(ii) Write a balanced chemical equation of another example of this type of reaction.
Answer:
(i) Combination reaction.
(ii) Another example of combination reaction is![]()
Question 11.
State the type of chemical reactions, represented by the following equations : (Board Term I, 2014)
(a) A + BC → AC + B
(b) A + B → C
(c) PQ + RS → PS + RQ
(d) A2O3 + 2B → B2O3 + 2A
Answer:
(a) Displacement reaction.
(b) Combination reaction.
(c) Double displacement reaction.
(d) Displacement reaction or redox reaction.
Question 12.
1 g of copper powder was taken in a China dish and heated. What change takes place on heating? When hydrogen gas is passed over this heated substance, a visible change is seen in it. Give the chemical equations of reactions, the name and the colour of the products formed in each case. (2020)
Answer:
When copper powder is heated in a China dish, the reddish brown surface of copper powder becomes coated with a black substance which is copper oxide.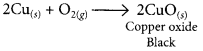
When hydrogen gas is passed over CuO, the black coating on the surface turned reddish brown due to the formation of Cu.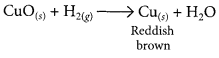
Question 13.
A compound ‘A’ is used in the manufacture of cement. When dissolved in water, it evolves a large amount of heat and forms compound ‘B’.
(i) Identify A and B.
(ii) Write chemical equation for the reaction of A with water.
(iii) List two types of reaction in which this reaction may be classified. (2020)
Answer:
(i) A is calcium oxide, CaO which is used in the manufacturing of cement.
B is calcium hydroxide Ca(OH)3.![]()
(iii) The given reaction is a combination reaction.
Example : NH3(g)(g) + HCl(g) → NH4Cl(s)
2NO(g) + 02(g) → 2NO2(g)
Question 14.
Mention with reason the colour changes observe when:
(i) silver chloride is exposed to sunlight.
(ii) copper powder is strongly heated in the presence of oxygen.
(iii) a piece of zinc is dropped in copper sulphate solution. (2020)
Answer:
(i) When white silver chloride is left exposed to sunlight, its colour changes to grey as it decomposes to silver in the presence of sunlight.![]()
This type of reaction is called photodecomposition reaction.
(ii) When copper powder is strongly heated in presence of oxygen, the reddish brown surface of copper powder becomes coated with a black substance which is copper oxide.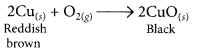
(iii) When a piece of zinc is dropped in copper sulphate solution, then the blue colour of copper sulphate fades gradually due to the formation of colourless zinc sulphate solution and reddish brown copper metal gets deposited on zinc piece.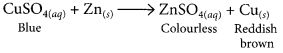
Question 15.
Lead nitrate solution is added to a test tube containing potassium iodide solution.
(a) Write the name and colour of the compound precipitated.
(b) Write the balanced chemical equation for the reaction involved.
(c) Name the type of this reaction justifying your answer. (2020)
Answer:
(a) When lead nitrate is added to potassium iodide then yellow precipitate of lead iodide is formed along with potassium nitrate.
(b) Balanced chemical reaction is as follows :![]()
(c) This type of reaction is called precipitation reaction in which one of the products formed is an insoluble substance or this is also called double displacement reaction.
Question 16.
2 g of silver chloride is taken in a China dish and the China dish is placed in sunlight for sometime. What will be your observation in this case? Write the chemical reaction involved in the form of a balanced chemical equation. Identify the type of chemical reaction. (Delhi 2019)
Answer:
Refer to answer 14(i).
Question 17.
Identify the type of reactions taking place in each of the following cases and write the balanced chemical equation for the reactions.
(a) Zinc reacts with silver nitrate to produce zinc nitrate and silver.
(b) Potassium iodide reacts with lead nitrate to produce potassium nitrate and lead iodide. (Delhi 2019)
Answer:
(a) It is a displacement reaction.![]()
(b) Refer to answer 15.
Question 18.
2 g of ferrous sulphate crystals are heated in a dry boiling tube. (Al 2019, Board Term 1, 2017, 2016)
(a) List any two observations.
(b) Name the type of chemical reaction taking place.
(c) Write balanced chemical equation for the reaction and name the products formed.
Answer:
(a) Ferrous sulphate crystals (FeSO4.7H2O) lose water when heated and the colour of the crystals changes. It then decomposes to ferric oxide (Fe2O3), sulphur dioxide (SO2) and sulphur trioxide (SO3) with a smell of burning sulphur.
(b) This is a thermal decomposition reaction.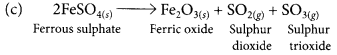
Question 19.
You might have noted that when copper powder is heated in a China dish, the reddish brown surface of copper powder becomes coated with a black substance. (AI 2019)
(a) Why has this black substance formed?
(b) What is the black substance?
(c) Write the chemical equation of the reaction that takes place.
(d) How can the black coating on the surface be turned reddish brown?
Answer:
(a) The black substance is formed because copper combines with oxygen.
(b) The black substance is copper oxide (CuO).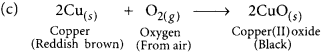
(d) The black coating on the surface can be turned reddish brown by passing hydrogen gas over the hot copper oxide.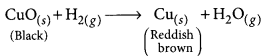
Question 20.
Decomposition reactions require energy either in the form of heat or light or electricity for breaking down the reactants. Write one equation each for decomposition reactions where energy is supplied in the form of heat, light and electricity. (2018)
Answer:
Decomposition reaction involving absorption of heat:![]()
Decomposition reaction involving absorption of light:![]()
Decomposition reaction involving absorption of electrical energy:![]()
Question 21.
Take 3 g of barium hydroxide in a test tube, now add about 2 g of ammonium chloride and mix the contents with the help of a glass rod. Now touch the test tube from outside.
(i) What do you feel on touching the test tube?
(ii) State the inference about the type of reaction occurred.
(iii) Write the balanced chemical equation of the reaction involved. (Board Term I, 2017)
Answer:
(i) When barium hydroxide is added into ammonium chloride, the bottom of test tube is found to be cooler.
(ii) It is an endothermic reaction.
(iii) Ba(OH)2 + 2NH4Cl → BaCl2 + 2NH4OH
Question 22.
(a) A solution of potassium chloride when mixed with silver nitrate solution, an insoluble white substance is formed. Write the chemical reaction involved and also mention the type of the chemical reaction.
(b) Ferrous sulphate when heated, decomposes with the evolution of a gas having a characteristic odour of burning sulphur. Write the chemical reaction involved and identify the type of reaction. (Board Term I, 2016)
Answer:
(a)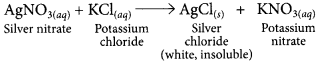
It is a double displacement reaction.
(b) Refer to answer 18(b) and (c).
Question 23.
Name the type of chemical reaction represented by the following equation: (Board Term I, 2016)
(i) CaO + H2O → Ca(OH)2
(ii) 3BaCl2 + Al2(SO4)3 → 2AlCl3 + 3BaSO4![]()
Answer:
(i) Combination reaction.
(ii) Precipitation reaction or double displacement reaction.
(iii) Thermal decomposition reaction.
Question 24.
What is a reduction reaction?
Identify the substances that are oxidised and the substances that are reduced in the following reactions. (Board Term I, 2015)
(a) Fe2O3 + 2Al → Al2O3 + 2Fe
(b) 2PbO + C → 2Pb + CO2
Answer:
Those reactions in which addition of hydrogen to a substance or removal of oxygen from a substance take place are called reduction reactions.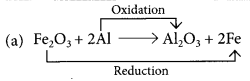
Fe2O3 is getting reduced to Fe and Al is getting oxidised to Al2O3.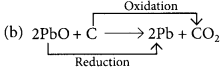
PbO is reduced to Pb and C is oxidised to CO2.
Question 25.
(a) Can a displacement reaction be a redox reaction? Explain with the help of an example.
(b) Write the type of chemical reaction in the following:
(i) Reaction between an acid and a base
(ii) Rusting of iron. (Board Term I, 2017)
Answer:
(a) Consider the following displacement reaction:
Zn(s)+ CuSO4(aq) → ZnSO4(aq) + Cu(s)
Here, Zn has changed into ZnSO4 (i.e., Zn2+ ions) by loss of electrons. Hence, Zn has been oxidised. CuSO4 (i.e., Cu2+) has changed into Cu by gain of electrons. Hence, CuSO4 has been reduced. Thus, the above reaction is a displacement reaction as well as a redox reaction.
(b) (i) Neutralisation reaction
(ii) Oxidation reaction.
Question 26.
Mention the type of chemical reaction that takes place when: (Board Term I, 2013)
(i) a magnesium ribbon is burnt in air.
(ii) limestone is heated.
(iii) silver bromide is exposed to sunlight.
(iv) electricity is passed through acidified water.
(v) ammonia and hydrogen chloride are mixed with each other.
Write the chemical equation for each reaction.
Answer:![]()
This is a combination reaction.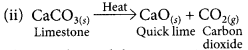
This is a thermal decomposition reaction.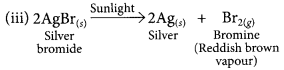
This is a photo decomposition reaction.![]()
This is electrolytic decomposition reaction.![]()
This is a combination reaction.
Question 27.
What happens when food materials containing fats and oils are left for a long time? List two observable changes and suggest three ways by which this phenomenon can be prevented. (2020)
Answer:
Food materials containing fats and oils change their taste and smell due to a process called rancidity. Rancidity is a process in which air reacts with fats and oils which changes the smell and taste of food.
Methods of prevention:
Vacuum packing,
refrigeration of food materials,
placing of food materials, away from direct sunlight.
Question 28.
(i) Why is respiration considered as an exothermic reaction?
(ii) Write chemical name and the formula of the brown gas produced during thermal decomposition of lead nitrate.
(iii) Why do chips manufactures flush bags of chips with gas such as nitrogen? (Board Term I, 2015)
Answer:
(i) The glucose produced in our body during digestion combines with oxygen in the cells of our body and provides energy. The special name of this reaction is respiration. Thus respiration is an exothermic process because energy is produced during this process.
C6H12O6(aq) + 6O2(g) → 6CO2(g) + 6H2O(l) + Energy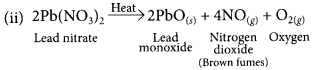
Brown gas evolved is nitrogen dioxide (NO2).
(iii) Chips manufacturers usually flush bags of chips with gas such as nitrogen because atmospheric oxygen can react with chips which may cause change in colour, change in taste. So to cut the contact between air and the chips, nitrogen gas is used which do prevent its oxidation.
Short Answer Type Questions[l] [2 Marks] -Year 2015
29.“We need to balance a skeltal chemical equation.” Give reason to justify the statement.
Answer:
Skeltal chemical equation are unbalanced. We need to balance chemical equation because of law of conservation of mass. It states that ‘matter can neither be created nor be destroyed’. Therefore chemical equation must be balanced in each and every chemical reaction.
| 30. Name the reducing agent in the following reaction: |
3MnO2 + 4Al———— > 3Mn + 2Al2O3
State which is more reactive, Mn or A1 and why?
Answer. ‘Al’ is reducing agent.
‘AT is more reactive than Mn v ‘Al’ displaces Mn from its oxide.
Short Answer Type Questions[ll] [3 Marks] -Year 2015
31.A Name the type of chemical reaction represented by the following equation: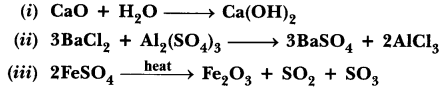
Answer.
(i) Combination reaction
(ii) Double displacement reaction (Precipitation reaction)
(iii) Decomposition reaction.
32. Write the chemical equation of the reaction in which the following changes have taken place with an example of each:
(i) Change in colour
(ii) Change in temperature
(iii) Formation of precipitate
Answer.
(i)Cu (s) + 2AgNO3 (aq)———–> Cu(NO3)2(aq) + 2Ag
The solution will become blue in colour and shiny silver metal will be deposited.
(ii) NaOH + HCl ———–> NaCl + H2O+ heat
The temperature will increase because heat will be evolved.
(iii) Pb(NO3)2 (aq) + 2KI (aq)———–> Pbl2 (s) + 2KNO3 (aq)
Yellow ppt
Yellow precipitate of Pbl2will be formed.
33.State the type of chemical reactions and chemical equations that take place in the following:
(i) Magnesium wire is burnt in air.
(ii) Electric current is passed through water.
(iii) Ammonia and hydrogen chloride gases’are mixed.
Answer.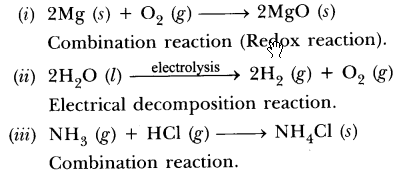
34. 2g of ferrous sulphate crystals are heated in a dry boiling tube.
(i) List any two observations.
(ii) Name the type of chemical reaction taking place.
(iii) ‘Write the chemical equation for the reaction.
Answer.
(i) •Green colour of Fe SO4 disappears and reddish brown solid is formed.
• Smell of burning sulphur.
(ii) Decomposition reaction![]()
Long Answer Type Questions [5 Marks] -Year 2015
35. (a) Define a balanced chemical equation. Why should an equation be balanced?
(b) Write the balanced chemical equation for the following reaction:
(i) Phosphorus burns in presence of chlorine to form phosphorus penta chloride.
(ii) Burning of natural gas.
(iii) The process of respiration.
Answer.
(a) Balanced chemical equation has an equal number of atoms of different elements in the reactants and products. According to law of conservation of mass, matter can neither be created nor be destroyed in a chemical reaction.
(b)(i) P4 (s) + 10Cl2 (g) ———> 4PCl5 (S)
(i)CH4 (g) + 2O2 (g) ———> CO2 (g) + 2H2O(l) + heat energy
(iii) C6H12O6 (s) + 6O2 (g) + 6H2O ———> 6CO2 (aq) + 12H2O (l) + energy
36.(a) Explain two ways by which food industries prevent rancidity.
(b) Discuss the importance of decomposition reaction in metal industry with three points.
Answer.
(a) (i) Rancidity can be prevented by adding antioxidants to food containing
fat and oil, e.g. butylated hydroxy anisole is added to butter as antioxidant.
(ii) It can be prevented by packaging fat and oil containing foods in nitrogen gas.
(b) (i) Molten NaCl is electrolytically decomposed to form sodium metal.
(ii) Aluminium metal is obtained by electric decomposition of bauxite ore mixed with cryolite.
(iii) Carbonate ores are thermally decomposed to give metal oxide which on reduction give metal.
Short Answer Type Question[I] [2 Marks] -Year 2014
37. What is observed when a solution of potassium iodide solution is added to a solution of lead nitrate? Name the type of reaction. Write a balanced chemical equation to represent the above chemical reaction.
Answer.Yellow precipitate of lead iodide is formed. It is precipitation reaction.
Pb( NO3)2 (aq) + 2KI (aq) —-> Pbl2 (s) + 2KNO3 (aq)
It is also called double displacement reaction.
short Answer Type Question[ll] [3 Marks] -Year 2014
38.Write chemical equation reactions taking place when carried out with the help of
(a) Iron reacts with steam
(b) Magnesium reacts with dil HCl
(c) Copper is heated in air.
Answer.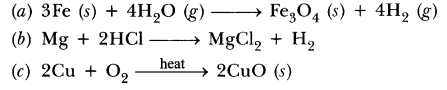
Long Answer Type Question [5 Marks] -Year 2014
39.(a) Write one example for each of decomposion reaction carried out with help of
(i) Electricity (ii) Heat (iii) Light
(b) Which of the following statements is correct and why copper can displace silver from silver nitrate and silver can displace copper from copper sulphate solution.
Answer.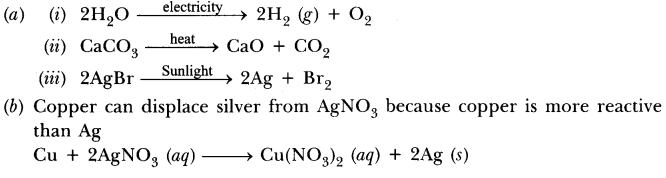
Short Answer Type Questions[ll] [3 Marks] -Year 2013
40. What is meant by skeltal type chemical equation? What does it represent? Using the equation for electrolytic decomposition of water, differentiate between a skeltal chemical equation and a balanced chemical equation.
Answer. The equations in which gaseous are written in atomic form instead of molecular form and equation is not balanced, are called skeltal type equation. They represent gaseous elements formed in atomic state and equation is not balanced
Short Answer Type Questions[l] [2 Marks]-Year 2012
41.Identify the type of reaction(s) in the following equations.
(i)CH4 + 2O2 CO2 + 2 H2O
(ii) Pb(NO3)2 + 2KI ——–>Pbl2 + 2KNOs
(iii) CaO + H2O ——–> Ca(OH)2
(iv) CuSO4 + Zn ——–> ZnSO4 + Cu
Answer.
(i) Combustion reaction and oxidation reaction.
(ii)Double displacement reaction and precipitation reaction.
(iii) Combination reaction.
(iv) Displacement reaction.
42.What is the colour of ferrous sulphate crystals? How does this colour change after heating?
Answer.The colour of ferrous sulphate is pale green. The colour changes to reddish brown on heating due to formation of iron (III) oxide.
Give an example each for thermal decomposition and photochemical decomposition reactions. Write relevant balanced chemical equations also.
Thermal decomposition reaction: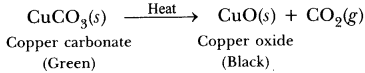
Photochemical decomposition reaction:![]()
43. Why does the colour of copper sulphate solution change when an iron nail is dipped in it? Write two observations.
Answer. It is because displacement reaction takes place.
Iron displaces copper from copper sulphate solution and forms pale green
coloured solution of FeS04 and reddish brown copper metal gets deposited.
Fe(s) + CuS04(aq) ——–> FeS04(aq) + Cu(s)
44. Translate the following statement into chemical equation and then balance it Barium chloride reacts with aluminium sulphate to give aluminium chloride and a precipitate of barium sulphate. State the two types in which this reaction can be classified.
Answer. 3BaCl2(aq) + A12(S04)3(aq) ——–> 3BaS04(s) + 2AlCl3(aq)
It can be classified as double displacement as well as precipitation reaction.
45. Why are decomposition reactions called the opposite of combination reactions? Write equations for these reactions.
Answer. In decomposition reaction, a compound is broken down into simpler compounds or elements, e.g.![]()
Combination reaction is a reaction in which two or more elements or compounds combine to form a new compound, e.g.![]()
Thus, decomposition and combination reactions are opposite to each other.
Short Answer Type Questions[ll] [3 Marks] -Year 2012
46. What is rancidity? Mention any two ways by which rancidity can be prevented.
Answer. The process in which taste and smell of food gets spoiled is called rancidity. It happens due to oxidation.
Prevention from rancidity:
(i) Antioxidants are added to fatty acids to prevent oxidation, e.g. chips are packed in presence of nitrogen gas which prevents spoilage by oxidation.
(ii)Food should be kept in airtight container in refrigerator.
47.Write balanced chemical equation for the reactions that take place during respiration. Identify the type of combination reaction that takes place during this process and justify the name. Give one more example of this type of reaction.
Answer. CgH1206 + 6O2 —————> 6CO2 + 6H20 + heat
It is an exothermic combination reaction because heat is evolved.
CH4(g) + 2O2(g) ————–>CO2 (g) + 2H20
Combustion of methane is another example of exothermic combination reaction.
48. What is redox reaction? Identify the substance oxidised and the substance reduced in the following reactions.
(i)2PbO + C —–> 2Pb + CO2
(ii)MnO2 + 4HCl —–> MnCl2 + 2H20 + Cl2
Answer. Those reactions in which oxidation and reduction takes place simultaneously are called redox reactions.
(i) PbO is getting reduced and C is getting oxidised.
(ii) MnOs is getting reduced and HCl is getting oxidised.
Very Short Answer Type Questions [1 Mark] -Year 2011
49.State one basic difference between a physical change and a chemical change.
Answer. In physical change, no new substance is formed, whereas in a chemical change, new substance(s) is/are formed.
50. What is meant by a chemical reaction?
Answer. The reaction representing a chemical change is called a chemical reaction.
35.AgN03(aq) + NaCl(aq)——————– > AgCl(s)4↓ + NaN03(aq)
FeS + H2S04————- > FeS04 + H2S↑
Consider the above mentioned two chemical equations with two different kinds of arrows (↑and ↓) along with product. What do these two different arrows indicate?
Ans,↑shows the gas is evolved whereas ↓shows insoluble substance (precipitate) is formed.
51. Hydrogen being a highly inflammable gas and oxygen being a supporter of combustion, yet water which is a compound made up of hydrogen and oxygen is used to extinguish fire. Why?
Answer. It is because properties of compound (H2O) are different from properties of its constituting elements, i.e. H2and O2.
Short Answer Type Questions[l] [2 Marks] -Year 2011
52.Using a suitable chemical equation, justify that some chemical reactions are determined by:
(i) change in colour, (ii) change in temperature.
Answer.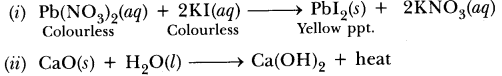
53.(a) A solution of substance ‘X’ is used for white washing. What is the substance ‘X’? State the chemical reaction of ‘X’ with water.
(b) Why does the colour of copper sulphate solution change when an iron nail is dipped in it?
Answer.
(a) ‘X’ is calcium oxide (CaO).
CaO(s) + H2O(l) —–> Ca(OH)2(aq) + heat
(a) It is because iron displaces copper from CuS04 to form FeS04 which is pale green.
Fe(s) + CUS04 (aq)—–> FeS04(aq) + Cu(s)
Blue Pale green
54.Write the balanced equation for the. following reaction and identify the type of reaction in each case.
(i) Potassium bromide + Barium iodide—-> Potassium iodide + Barium bromide.
(ii) Hydrogen(g) + Chlorine(g)—-> Hydrogen chloride(g)
Answer.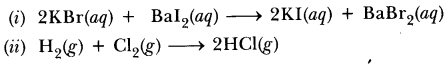
55. A zinc plate was put into a solution of copper sulphate kept in a glass container. It was found that blue colour of the solution gets fader and fader with the passage of time. After few days, when zinc plate was taken out of the solution, a number of holes were observed on it.
(i) State the reason for changes observed on the zinc plate.
(ii) Write the chemical equation for the reaction involved.
Answer.
(i) It is because zinc has displaced copper from CuS04. Zinc metal has been used to form zinc sulphate, therefore, number of holes were observed.![]()
56. A white salt on heating decomposes to give brown fumes and a residue is left behind.
(i) Name the salt.
(ii) Write the equation for the decom-position reaction.
Answer.
(i) Lead nitrate is white salt.![]()
57. When a solution of potassium iodide is added to a solution of lead nitrate in a test tube, a reaction takes place.
(a) What type of reaction is this?
(b) Write a balanced chemical equation to represent the above reaction.
Answer.
(a) Double displacement as well as precipitation reaction.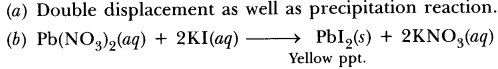
58. Write balanced equations for the following mentioning the type of reaction involved.
(i) Aluminium + Bromine —–> Aluminium bromide
(ii) Calcium carbonate—–> Calcium oxide + Carbon dioxide
(iii) Silver chloride—–>Silver + Chlorine
Answer.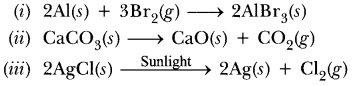
59.(a) Why is respiration considered as an exothermic reaction?
(b) Define the terms oxidation and reduction.
(c) Identify the substance that is oxidised and reduced in the following reaction.![]()
Answer. (a) It is because heat is evolved during respiration.
(b) Oxidation is a process in which O2 is added or H2 is removed or loss of electrons take place. Reduction is a process in which H2 is added or O2. is removed or gain of electrons take place.
(c) Zn is getting oxidised, CuO is getting reduced.
60.You might have noted that when copper powder is heated in a china dish, the surface of copper powder becomes coated with a black colour substance.
(i) How has this black coloured substance formed?
(ii) What is that black substance?
(iii) Write the chemical equation of the reaction that takes place.
Answer.
(i) Copper reacts with oxygen to form copper oxide which is black, i.e. oxidation of copper takes place.
(ii)Copper oxide![]()
Very Short Answer Type Questions [1 Mark] -Year 2010
61. What happens chemically when quicklime is added to water filled in a bucket?
Answer. Quicklime reacts with water to form slaked lime and produces lot of heat and hissing sound.
62. On what basis is a chemical equation balanced?
Answer. A chemical reaction is balanced on the basis of law of conservation of mass.
63. What change in colour is observed when white silver chloride is left exposed to sunlight? State the type of chemical reaction in this change.
Answer. Silver chloride becomes grey. It is a photochemical decomposition reaction.
64. Write a balanced chemical equation for the reaction between sodium chloride
and silver nitrate indicating the physical state of the reactants and the products.
Answer.![]()
Short Answer Type Questions[l] [2 Marks]
65. What happens when an aqueous solution of sodium sulphate reacts with an aqueous solution of barium chloride? State the physical conditions of reactants in which the reaction between them will not take place. Write the balanced chemical equation for the reaction and name the type of reaction.
Answer. White precipitate of barium sulphate is formed.
If both reactants are in solid state, then the reaction will not take place between them.![]()
It is a double displacement as well as a precipitation reaction.
66. What is a redox reaction? When a magnesium ribbon burns in air with a dazzling flame and forms a white ash, is magnesium oxidised or reduced? Why?
Answer. The reactions in which oxidation (loss of electrons) and reduction (gain of electrons) take place simultaneously are called redox reactions.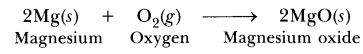
Magnesium is getting oxidised because it is losing electrons to form Mg2+ and oxygen is gaining electrons to form O2-, therefore it is getting reduced.
67. Write any two observations in an activity which may suggest that a chemical reaction has taken place. Give an example in support of your answer.
Answer. Any two of these observations will suggest chemical reaction has taken place.
(i) Change in state.
(ii)Change in colour.
(iii) Evolution of gas.
(iv)Change in temperature.
For example, lead nitrate is white crystalline solid which on heating gives yellowish brown solid (lead monoxide). A brown gas and a colourless gas is also evolved. It shows chemical reaction has taken place.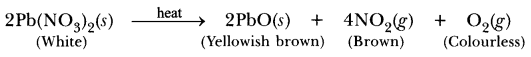
Very Short Answer Type Questions [1 Mark] -Year 2009
68.In electrolysis of water, why is the volume of gas collected over one electrode double that of gas collected over the other electrode?
Answer.It is because water contains hydrogen and oxygen in the ratio of 2 : 1.
69.Balance the following chemical equations.
Answer.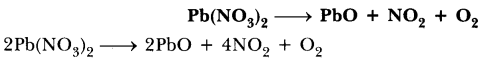
Short Answer Type Questions[l] [2 Marks] -Year 2009
70. Name the products formed on strongly heating ferrous sulphate crystals. What type of chemical reaction occurs in this change?
Answer.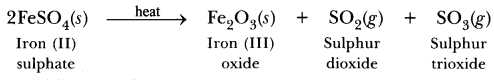
It is decomposition reaction.
71. What is an oxidation reaction? Give an example of oxidation reaction. Is oxidation an exothermic or an endothermic reaction?
Answer. The reaction in which oxygen or electronegative element is added or hydrogen or electropositive element is removed or loss of electrons takes place, is called an oxidation reaction, e.g. ,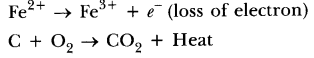
Oxidation reactions are mostly exothermic in nature because heat is evolved in this process.
72. Describe an activity to demonstrate the change that takes place when white silver chloride is kept in sunlight. State the type of chemical reaction which takes place.
Answer.
Aim: To demonstrate the change that takes place when white silver chloride is kept in sunlight.
Materials Required: AgNO3(aq), NaCl(aq), test tubes.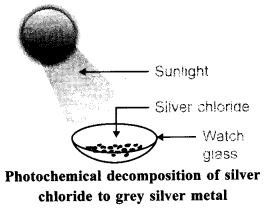
Procedure:
1. Take 5 ml of silver nitrate solution in a test tube.
2. Prepare sodium chloride solution in another test tube.
3. Add sodium chloride solution into test tube containing silver nitrate solution.
4. Observe the colour of silver chloride formed chloride to grey silver metal Dry it with the help of filter papers and place it on the watch glass.
5. Place the watch glass under sunlight for sometime.
6. Observe the colour of the silver chloride after sometime. Observation: White silver chloride turns grey in sunlight because silver metal is formed.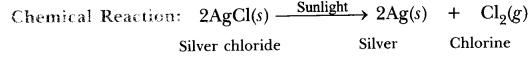
Explanation: Silver chloride is photosensitive. It decomposes in presence of sunlight to form silver metal and chlorine gas.
Conclusion: Decomposition of silver chloride in presence of sunlight is photochemical decomposition reaction.
73.When magnesium ribbon burns in air or oxygen, a product is formed. State the type of chemical reaction and name the product formed in the reaction. Write balanced chemical equation of this reaction.
Answer.
![]()
The type of reaction is combination reaction and the product formed is magnesium oxide.
74.Distinguish between a displacement reaction and a double displacement reaction. Identify the displacement and the double displacement reaction from the following reactions.
Answer.
Displacement reaction is a reaction in which more reactive metal can displace less reactive metal from its salt solution.
Double displacement reaction are those reactions in which compounds exchange their ions to form two new compounds (?) Double displacement reaction (ii) Displacement reaction
75.When you have mixed the solutions of lead(II) nitrate and potassium iodide,
(i) what was the colour of the precipitate formed and can you name the precipitate?
(ii) write the balanced chemical equation for this reaction.
(iii) is this also a double displacement reaction?
Answer.
(i) The colour of the precipitate is yellow. The name of compound formed as a precipitate is Pbl2 (lead iodide).![]()
(iii) Yes, it is also a double displacement reaction.
76.What do you mean by exothermic and endothermic reactions? Give examples.
Answer.Exothermic reactions are those in which heat is evolved, e.g.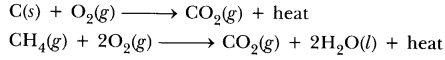
Endothermic reactions are those reactions in which heat is absorbed, e.g.